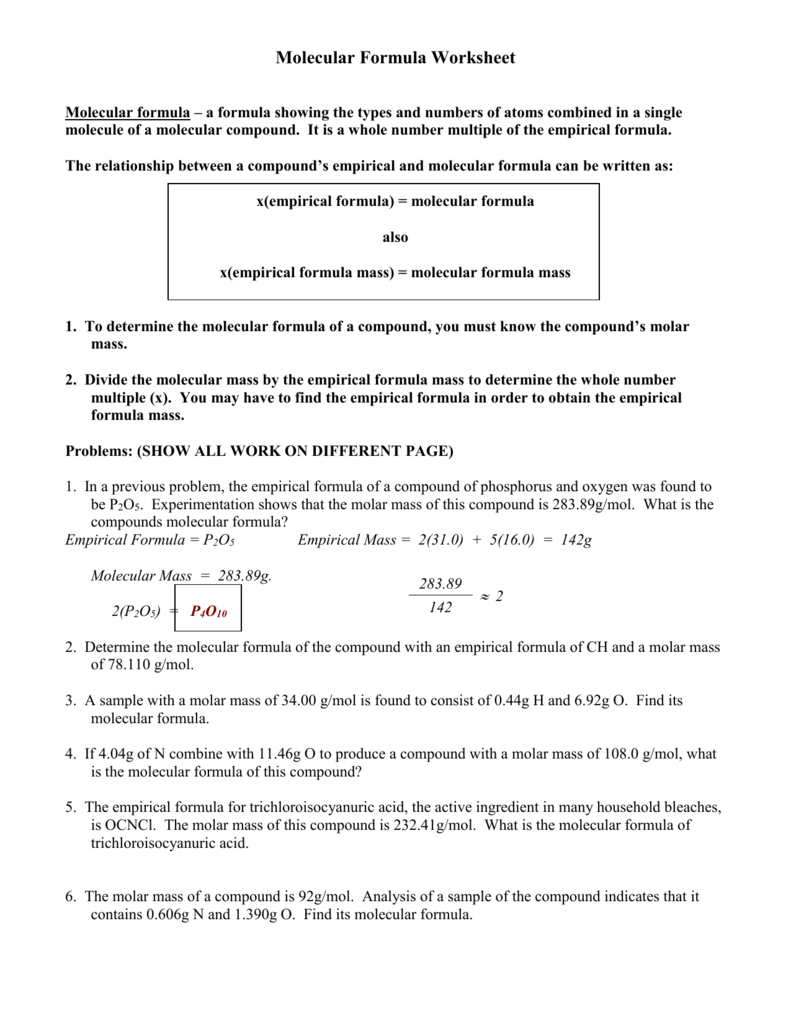
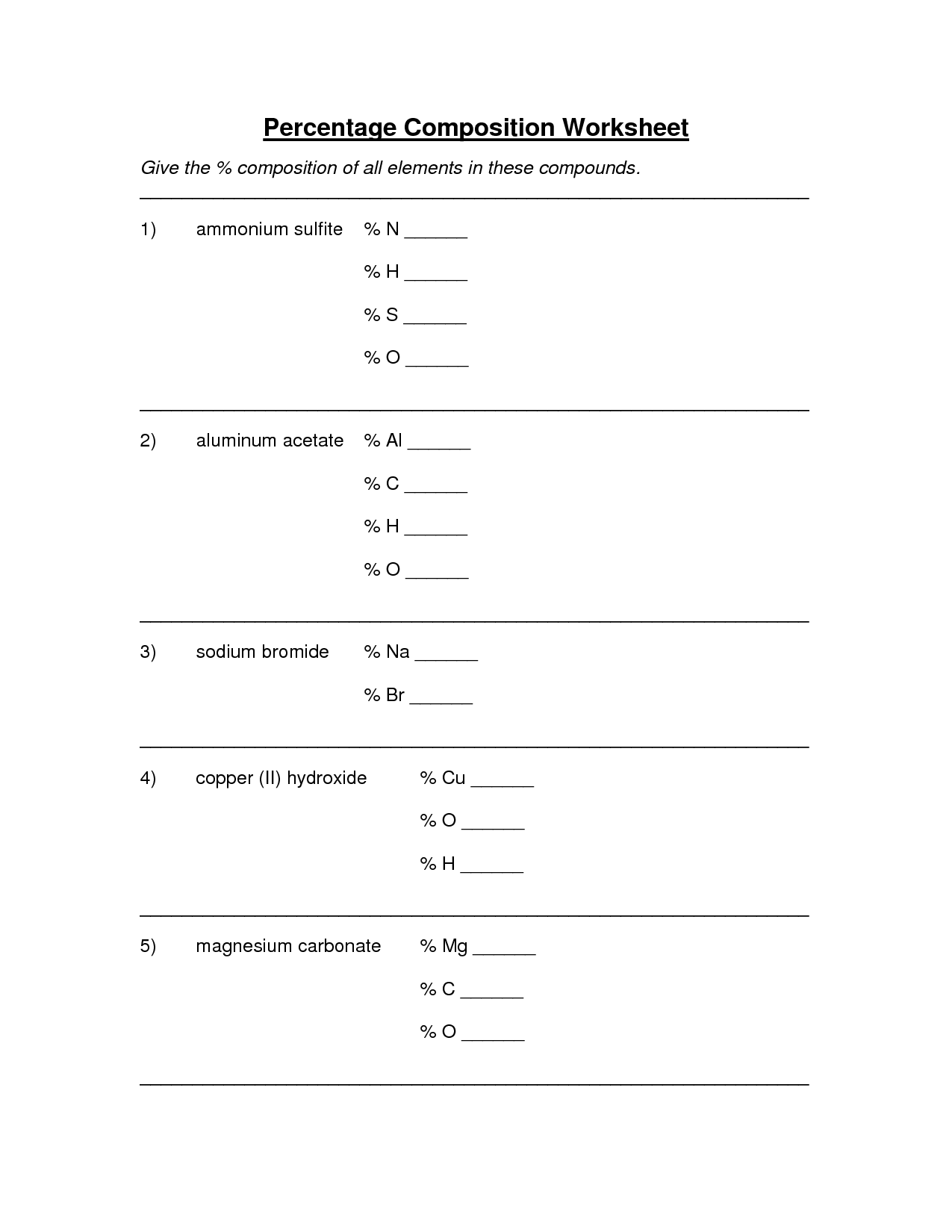
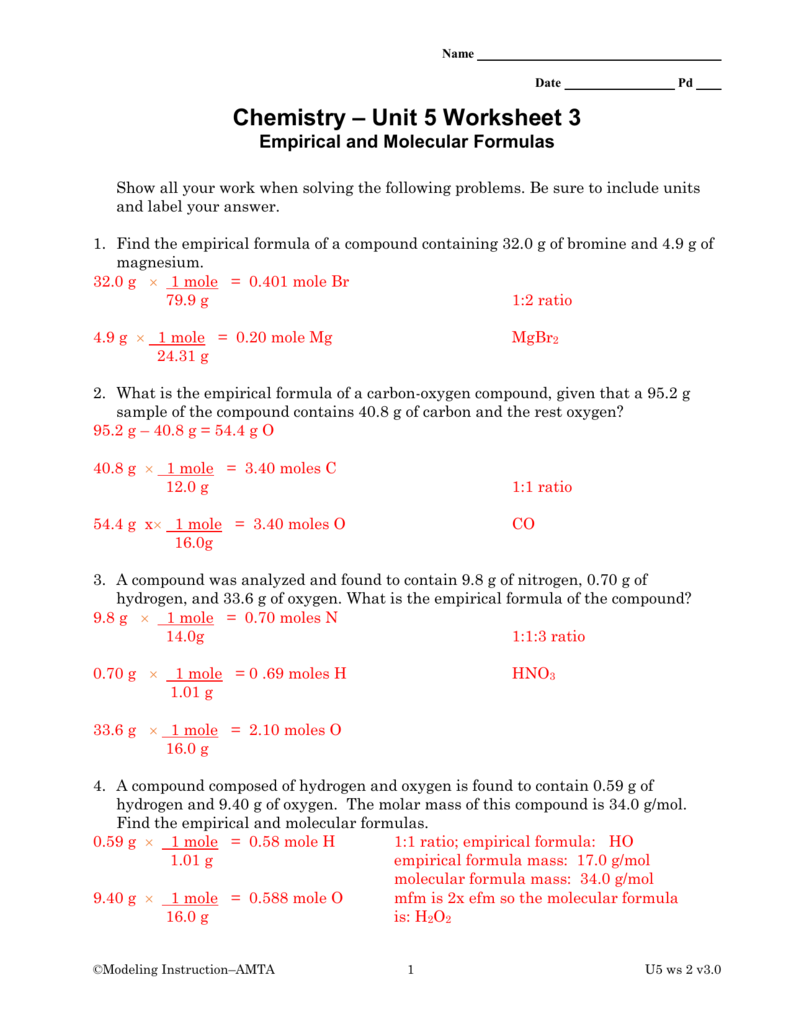
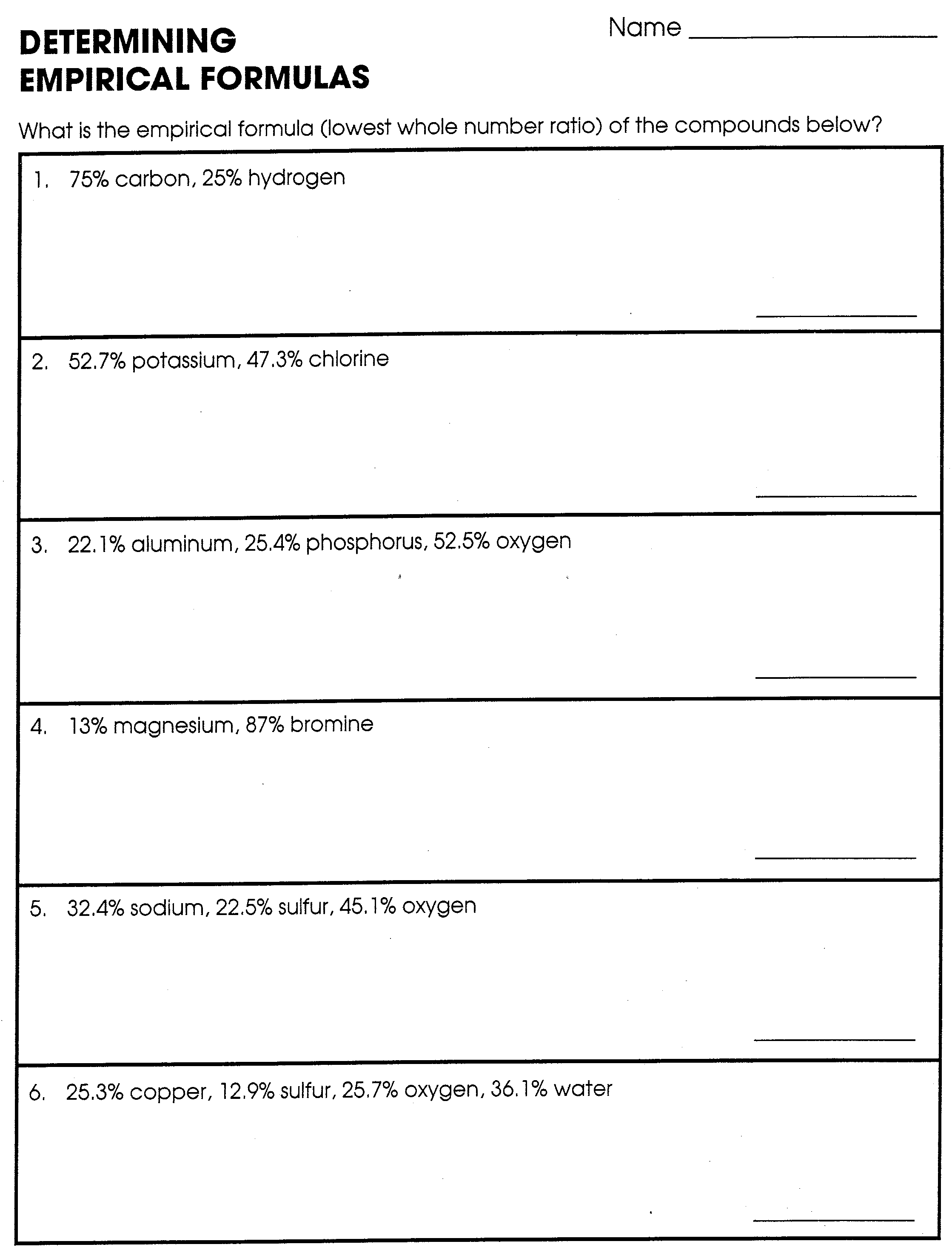
Percent Composition Empirical And Molecular Formulas Worksheet Answers
Percent Composition Empirical And Molecular Formulas Worksheet Answers - A compound is found to contain 36.5% na, 25.4% s, and 38.1% o. The first worksheet gives your students practice with finding the percent composition of a compound from a given formula, finding percent. Web guided worksheet that reviews determining percent composition of a compound, finding hydrate formulas based on mass from a dehydration reaction, using percent. Web 230 + results sort by: Web up to 24% cash back % composition, empirical formula and molecular formula worksheet a sample of an unknown compound with a mass of 0.847 g has the following. A sample of an unknown substance is found to have 49.37 g c, 4.14 g h and 21.86 g o. A compound with an empirical formula of c 4 h 4 o and a molar mass of 136 grams per mole. Web what is the molecular formula? Web the empirical formula only tells us the ratios of atoms in a compound, but the molecular formula tells us the actual number of atoms in the chemical formula. Web percent composition and molecular formula worksheet solutions 1) what’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0%. Write the empirical formula for the following compounds. Web up to 24% cash back % composition, empirical formula and molecular formula worksheet a sample of an unknown compound with a mass of 0.847 g has the following. The percentage composition of acetic acid is. 43% c and 57% o 40.3% k, 26.7% cr, and 33.0% o 32.0% c, 42.6% o,. Web this is a package of two worksheets. Web up to 24% cash back % composition, empirical formula and molecular formula worksheet a sample of an unknown compound with a mass of 0.847 g has the following. Web empirical and molecular formula worksheet show work on a separate sheet of paper. A sample of an unknown substance is found to. Percent composition of c = 65.5%, h = 5.5%, o = 29.0% for determining the empirical formula, no. Find the empirical formula of a compound that is 53.7% iron and 46.3%. The percentage composition of acetic acid is found to be 39% c, 6% h, and 53%. List percent composition, empirical & molecular formulas mega unit bundle created by chemistry. Write the empirical formula for the following compounds. Add art and creativity to your review lesson!. Web write the molecular formulas of the following compounds: A sample of an unknown substance is found to have 49.37 g c, 4.14 g h and 21.86 g o. Web steps to writing molecular formulas. Web guided worksheet that reviews determining percent composition of a compound, finding hydrate formulas based on mass from a dehydration reaction, using percent. Percent composition of c = 65.5%, h = 5.5%, o = 29.0% for determining the empirical formula, no. 43% c and 57% o 40.3% k, 26.7% cr, and 33.0% o 32.0% c, 42.6% o, 18.7% n, and. List percent composition, empirical & molecular formulas mega unit bundle created by chemistry corner everything you need for. 43% c and 57% o 40.3% k, 26.7% cr, and 33.0% o 32.0% c, 42.6% o, 18.7% n, and the remainder h 31.9% k, 28.9% cl, and the remainder o 52.8% sn, 12.4% fe, 16.0% c, and 18.8% n Calculate the molar. Find the percent composition of each element in the. Add art and creativity to your review lesson!. The percentage composition of acetic acid is. Web what is the molecular formula? A compound is found to contain 36.5% na, 25.4% s, and 38.1% o. The percentage composition of acetic acid is. Web this is a package of two worksheets. Web the empirical formula only tells us the ratios of atoms in a compound, but the molecular formula tells us the actual number of atoms in the chemical formula. Web empirical and molecular formula worksheet show work on a separate sheet of paper. Web a. Web a compound with an empirical formula of c 2 h 8 n and a molar mass of 46 grams per mole. Web percent composition and molecular formula worksheet solutions 1) what’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0%. Web determine the empirical formula for each compound whose percentage composition is shown below. The. Find the percent composition of each element in the. 43% c and 57% o 40.3% k, 26.7% cr, and 33.0% o 32.0% c, 42.6% o, 18.7% n, and the remainder h 31.9% k, 28.9% cl, and the remainder o 52.8% sn, 12.4% fe, 16.0% c, and 18.8% n A sample of an unknown substance is found to have 49.37 g. A sample of an unknown substance is found to have 49.37 g c, 4.14 g h and 21.86 g o. Web this is a package of two worksheets. Web guided worksheet that reviews determining percent composition of a compound, finding hydrate formulas based on mass from a dehydration reaction, using percent. The percentage composition of acetic acid is. 43% c and 57% o 40.3% k, 26.7% cr, and 33.0% o 32.0% c, 42.6% o, 18.7% n, and the remainder h 31.9% k, 28.9% cl, and the remainder o 52.8% sn, 12.4% fe, 16.0% c, and 18.8% n Web up to 24% cash back % composition, empirical formula and molecular formula worksheet a sample of an unknown compound with a mass of 0.847 g has the following. Web determine the empirical formula for each compound whose percentage composition is shown below. A compound with an empirical formula of c2h&n and a molar mass of 46 grams per mole. Find the percent composition of each element in the. Web steps to writing molecular formulas. The first worksheet gives your students practice with finding the percent composition of a compound from a given formula, finding percent. Of c atoms = 65.5/12.01 = 5.45 no. Web what is the molecular formula? Web a compound with an empirical formula of c 2 h 8 n and a molar mass of 46 grams per mole. Web empirical and molecular formula worksheet show work on a separate sheet of paper. Percent composition of c = 65.5%, h = 5.5%, o = 29.0% for determining the empirical formula, no. Web 230 + results sort by: The percentage composition of acetic acid is found to be 39% c, 6% h, and 53%. Calculate the molar mass of the empirical formula. Web percent composition and molecular formula worksheet solutions 1) what’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0%. Web write the molecular formulas of the following compounds: 43% c and 57% o 40.3% k, 26.7% cr, and 33.0% o 32.0% c, 42.6% o, 18.7% n, and the remainder h 31.9% k, 28.9% cl, and the remainder o 52.8% sn, 12.4% fe, 16.0% c, and 18.8% n Web percent composition and molecular formula worksheet solutions 1) what’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0%. Write the empirical formula for the following compounds. Web the empirical formula only tells us the ratios of atoms in a compound, but the molecular formula tells us the actual number of atoms in the chemical formula. Add art and creativity to your review lesson!. If you are not given the empirical formula, you must figure out what it is. The first worksheet gives your students practice with finding the percent composition of a compound from a given formula, finding percent. Web what is the molecular formula? List percent composition, empirical & molecular formulas mega unit bundle created by chemistry corner everything you need for. Web determine the empirical formula for each compound whose percentage composition is shown below. A sample of an unknown substance is found to have 49.37 g c, 4.14 g h and 21.86 g o. Find the percent composition of each element in the. Percent composition of c = 65.5%, h = 5.5%, o = 29.0% for determining the empirical formula, no. The percentage composition of acetic acid is found to be 39% c, 6% h, and 53%. Web empirical and molecular formula worksheet show work on a separate sheet of paper.Empirical And Molecular Formulas Worksheet
14 Best Images of Chemistry Molecular Formula Worksheet Answers
Empirical and Molecular Formulas
worksheet. Empirical Formula Worksheet. Grass Fedjp Worksheet Study Site
Percent Composition and Molecular Formula Worksheet
Empirical and Molecular Formulas Worksheet for 9th 12th Grade
percent composition and empirical formula worksheet
Empirical And Molecular Formulas Worksheet
Percent Composition and Molecular Formula Worksheet
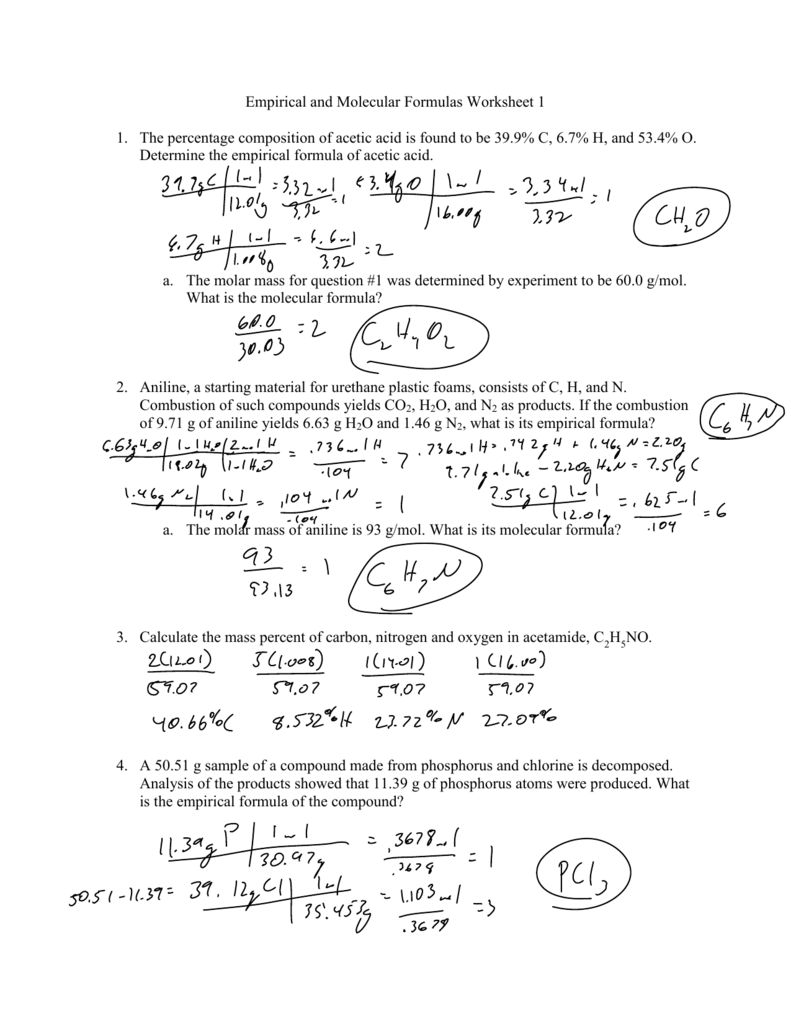
Empirical and Molecular Formulas Worksheet 1 1. The percentage
Web A Compound With An Empirical Formula Of C 2 H 8 N And A Molar Mass Of 46 Grams Per Mole.
Find The Empirical Formula Of A Compound That Is 53.7% Iron And 46.3%.
A Compound Is Found To Contain 36.5% Na, 25.4% S, And 38.1% O.
Web 230 + Results Sort By:
Related Post: