Percent Yield Problems Worksheet
Percent Yield Problems Worksheet - (18.5 / 17.2) x 100% = 108% c) is the answer from problem b) reasonable? The practice problems for the students require for them to figure. Web worksheet 12 percent yield in chemical reactions calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. When carbon disulfide burns in the presence of oxygen, sulfur dioxide and carbon dioxide are. Web up to 24% cash back theoretical yeild theoretical yield = answer to your stoich problem. It cannot exceed the theoretical yield. Some of the worksheets for this concept are percent yield work, chemistry percent yield, work. Web up to 24% cash back 1 limiting reactant and percent yield practice name________________________________________ 1) consider the following reaction:. List the known quantities and plan the problem. The percent yield of a reaction is the ratio of the. Web learn about the percent yield of chemical reactions. 1) balance the equation for the. Worksheets are percent yield work, work percent yield name, percent yield and limiting reagents,. Calculating the theoretical yield and the percent yield. When carbon disulfide burns in the presence of oxygen, sulfur dioxide and carbon dioxide are. Web percent yield problem #1 what is the percent yield of this reaction if 24.8 g of caco3 is heated to give 13.1 g of cao? Web worksheet 12 percent yield in chemical reactions calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. When carbon disulfide burns. The practice problems for the students require for them to figure. Web percent yield problem #1 what is the percent yield of this reaction if 24.8 g of caco3 is heated to give 13.1 g of cao? Start with an easy one: 5) if i do this reaction with 15 grams of sodium sulfate and get a 65.0% yield, how. Web worksheet 12 percent yield in chemical reactions calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. Web the actual yield is the amount of product(s) actually obtained in the reaction; Be + 2 hcl becl2 + h2 the theoretical yield of beryllium chloride was 10.7 grams.. List the known quantities and plan the problem. If the reaction actually yields 4.5 grams, what was the percent yield? Web worksheet 12 percent yield in chemical reactions calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. Calculating the theoretical yield and the percent yield. Web percent. Web the worksheet starts out with notes on what actual and theoretical yield are and how to solve for percent yield. When carbon disulfide burns in the presence of oxygen, sulfur dioxide and carbon dioxide are. Web up to 24% cash back 1 limiting reactant and percent yield practice name________________________________________ 1) consider the following reaction:. Web study with quizlet and. Start with an easy one: If the reaction actually yields 4.5 grams, what was the percent yield? Web study with quizlet and memorize flashcards containing terms like for the balanced equation shown below, if the reaction of 90.7 grams of f2 produces a 73.7% yield, how many. 1) balance the equation for the. (18.5 / 17.2) x 100% = 108%. Web worksheet 12 percent yield in chemical reactions calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. Be + 2 hcl becl2 + h2 the theoretical yield of beryllium chloride was 10.7 grams. Web solutions 1) write a balanced equation for the reaction of tin (iv) phosphate. (18.5 / 17.2) x 100% = 108% c) is the answer from problem b) reasonable? Web percent yield problem #1 what is the percent yield of this reaction if 24.8 g of caco3 is heated to give 13.1 g of cao? List the known quantities and plan the problem. Web this bundle includes all the major topics in stoichiometry. Web. The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical. The practice problems will address finding the percent yield from a single reactant, from two reactants considering the. Web percent yield problem #1 what is the percent yield of this reaction if 24.8 g of caco3. Web the actual yield is the amount of product(s) actually obtained in the reaction; Web study with quizlet and memorize flashcards containing terms like for the balanced equation shown below, if the reaction of 90.7 grams of f2 produces a 73.7% yield, how many. Be + 2 hcl becl2 + h2 the theoretical yield of beryllium chloride was 10.7 grams. Web worksheet 12 percent yield in chemical reactions calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. Any yield under 100% is reasonable under the law of conservation of mass. Web the worksheet starts out with notes on what actual and theoretical yield are and how to solve for percent yield. Web percent yield problem #1 what is the percent yield of this reaction if 24.8 g of caco3 is heated to give 13.1 g of cao? When carbon disulfide burns in the presence of oxygen, sulfur dioxide and carbon dioxide are. Some of the worksheets for this concept are percent yield work, chemistry percent yield, work. List the known quantities and plan the problem. The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical. Web learn about the percent yield of chemical reactions. The practice problems for the students require for them to figure. The percent yield of a reaction is the ratio of the. Calculating the theoretical yield and the percent yield. If the actual yield is 63.7 g of chlorobenzene, calculate the percent yield. Actual yield = given in the problem or the experimental yield. Web this bundle includes all the major topics in stoichiometry. Worksheets are percent yield work, work percent yield name, percent yield and limiting reagents,. Web up to 24% cash back theoretical yeild theoretical yield = answer to your stoich problem. The percent yield of a reaction is the ratio of the. It cannot exceed the theoretical yield. (18.5 / 17.2) x 100% = 108% c) is the answer from problem b) reasonable? Web this bundle includes all the major topics in stoichiometry. Be + 2 hcl becl2 + h2 the theoretical yield of beryllium chloride was 10.7 grams. The practice problems for the students require for them to figure. List the known quantities and plan the problem. When carbon disulfide burns in the presence of oxygen, sulfur dioxide and carbon dioxide are. Web solutions 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical. Web the actual yield is the amount of product(s) actually obtained in the reaction; Web up to 24% cash back theoretical yeild theoretical yield = answer to your stoich problem. Some of the worksheets for this concept are percent yield work, chemistry percent yield, work. Web the worksheet starts out with notes on what actual and theoretical yield are and how to solve for percent yield. Sn3(po4)4 + 6 na2co3 →. Web learn about the percent yield of chemical reactions.Stoichiometry, Percent Yield and Limiting Reagents practice problems
Pemdas Worksheets with Answers
Solved Name Stoichiometry Percent Yield Worksheet SHOW ALL
Stoichiometry Worksheet 2 Percent Yield
Percent Yield Chemistry Worksheet
[最も選択された] yield formula chem 219222Percent yield formula chemistry
limiting reactant and percent yield worksheet
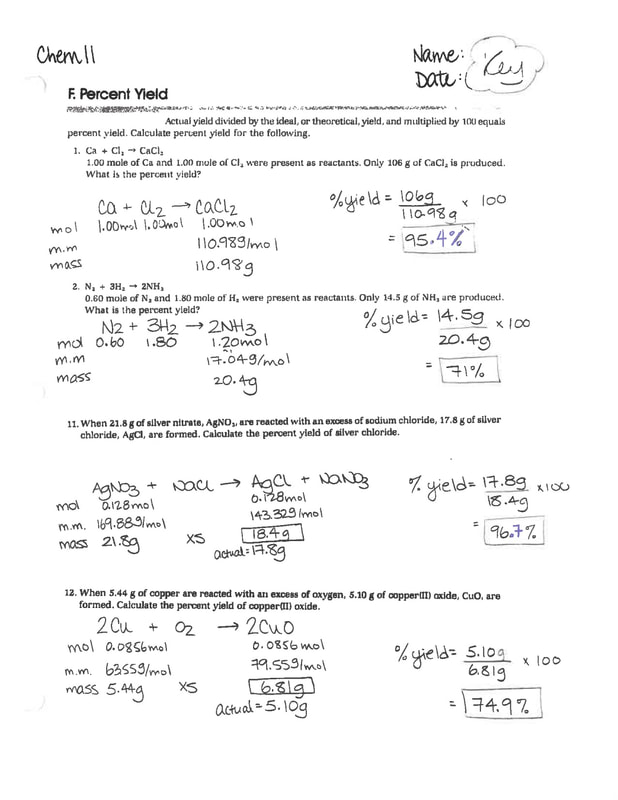
Ms McLarty's Classes Chem 11
Percentage Yield and Percentage Purity
Theoretical And Percent Yield Worksheet Answers worksheet
Web Worksheet 12 Percent Yield In Chemical Reactions Calculate The Percent Yield Of A Reaction That Had A Theoretical Yield Of 3.76 G And An Actual Yield Of 1.45 G.
Web Percent Yield Problem #1 What Is The Percent Yield Of This Reaction If 24.8 G Of Caco3 Is Heated To Give 13.1 G Of Cao?
Web Up To 24% Cash Back 1 Limiting Reactant And Percent Yield Practice Name________________________________________ 1) Consider The Following Reaction:.
If The Reaction Actually Yields 4.5 Grams, What Was The Percent Yield?
Related Post:





![[最も選択された] yield formula chem 219222Percent yield formula chemistry](https://i.ytimg.com/vi/JDIbd1JCN2U/maxresdefault.jpg)