Percent Yield Worksheet Answer Key
Percent Yield Worksheet Answer Key - Etical yield in grams of co. (18.5 / 17.2) x 100% = 108% c) is the answer from problem b) reasonable? Etical yield in grams of agno 3. To answer this, we just use the following equation: 2) if i perform this. Web percent, actual, and theoretical yield 1. Web worksheets are percent yield work, work percent yield name, percent yield and limiting reagents, chem1001 work 5 yields model 1 limiting reagents, math 120 section. If the actual yield is 63.7 g of chlorobenzene, calculate the percent yield. What is the theoretical yield if 45.6 g of benzene react? The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical. What is my theoretical yield of lithium chloride? Web calculate the theoretical yield and percentage yield of fe2o3(s). 2) if i perform this. Exercises add to my workbooks (8). What is the limiting reactant when 154 g of ag reacts with 189 g o. Web up to 24% cash back answer sheet 1) consider the following reaction: Web theoretical yield/ percent yield. 3 nh 4 no 3 + na 3 po 4 (nh 4 3 po 4 + 3 nano 3 answer the questions above, assuming we started with 30. 2) if i perform this. Web percent yield worksheet 1) write the equation for. If the actual yield is 63.7 g of chlorobenzene, calculate the percent yield. 3 nh 4 no 3 + na 3 po 4 (nh 4 3 po 4 + 3 nano 3 answer the questions above, assuming we started with 30. 2) if i perform this. Web percent, actual, and theoretical yield 1. What is the theoretical yield if 45.6. Web worksheets are percent yield work, work percent yield name, percent yield and limiting reagents, chem1001 work 5 yields model 1 limiting reagents, math 120 section. I began this reaction with 20 grams of lithium hydroxide. Web up to 24% cash back created date: Web calculate the theoretical yield and percentage yield of fe2o3(s). Calculate the percent yield of a. 3 nh 4 no 3 + na 3 po 4 (nh 4 3 po 4 + 3 nano 3 answer the questions above, assuming we started with 30. If the actual yield is 63.7 g of chlorobenzene, calculate the percent yield. Calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield. Web theoretical yield/ percent yield. Web percent yield worksheet 1) write the equation for the reaction of iron (iii) phosphate with sodium sulfate to make iron (iii) sulfate and sodium phosphate. _____ k2ptcl4 + __ 2 ___ nh3 g. Web up to 24% cash back created date: Exercises add to my workbooks (8). Calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. Lioh + kcl licl + koh a. What is my theoretical yield of lithium chloride? Exercises add to my workbooks (8). _____ k2ptcl4 + __ 2 ___ nh3 g. Exercises add to my workbooks (8). When carbon disulfide burns in the. Web up to 24% cash back created date: Lioh + kcl licl + koh a. Web up to 24% cash back answer sheet 1) consider the following reaction: Any yield over 100% is a violation of the law of conservation of mass. Web worksheets are percent yield work, work percent yield name, percent yield and limiting reagents, chem1001 work 5 yields model 1 limiting reagents, math 120 section. What is the limiting reactant when 154 g of ag reacts with 189 g o. Web calculate the theoretical yield. If the actual yield is 63.7 g of chlorobenzene, calculate the percent yield. Calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. 2) if i perform this. Web theoretical yield/ percent yield. (18.5 / 17.2) x 100% = 108% c) is the answer from problem b) reasonable? I began this reaction with 20 grams of lithium hydroxide. If the actual yield is 63.7 g of chlorobenzene, calculate the percent yield. _____ k2ptcl4 + __ 2 ___ nh3 g. Web percent, actual, and theoretical yield 1. Exercises add to my workbooks (8). Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. When carbon disulfide burns in the. Web percent yield worksheet 1) write the equation for the reaction of iron (iii) phosphate with sodium sulfate to make iron (iii) sulfate and sodium phosphate. Web calculate the theoretical yield and percentage yield of fe2o3(s). 2) if i perform this. Lioh + kcl licl + koh a. Web theoretical yield/ percent yield. Web worksheets are percent yield work, work percent yield name, percent yield and limiting reagents, chem1001 work 5 yields model 1 limiting reagents, math 120 section. What is my theoretical yield of lithium chloride? What is the limiting reactant when 154 g of ag reacts with 189 g o. 4fes(s)+ 7o (g)→ 2fe2o3 (s)+ 4so2 (g) mfes = 87.91 g/mol mfe2o3 = 159.69 nfes = 16.1 g 87.91 g/mol. Web up to 24% cash back answer sheet 1) consider the following reaction: (18.5 / 17.2) x 100% = 108% c) is the answer from problem b) reasonable? Any yield over 100% is a violation of the law of conservation of mass. To answer this, we just use the following equation: Web percent, actual, and theoretical yield 1. To answer this, we just use the following equation: _____ k2ptcl4 + _____ nh3 g _____ pt (nh3)2cl2 + _____ kcl. (18.5 / 17.2) x 100% = 108% c) is the answer from problem b) reasonable? Web up to 24% cash back created date: When carbon disulfide burns in the. Web up to 24% cash back answer sheet 1) consider the following reaction: If the actual yield is 63.7 g of chlorobenzene, calculate the percent yield. Exercises add to my workbooks (8). A student used 1.34 g of silver to. I began this reaction with 20 grams of lithium hydroxide. Lioh + kcl licl + koh a. Web theoretical yield/ percent yield. The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical. What is the limiting reactant when 154 g of ag reacts with 189 g o. 3 nh 4 no 3 + na 3 po 4 (nh 4 3 po 4 + 3 nano 3 answer the questions above, assuming we started with 30.Limiting Reagents and Percentage Yield Worksheet answers.doc Zinc
14 Stoichiometry Worksheet 2 Answer Key /
Limiting Reactant And Percent Yield Practice Worksheet Answer Key
Limiting Reagents And Percentage Yield Worksheet Answers
Limiting Reactant And Percent Yield Worksheet Answer Key —
Theoretical And Percent Yield Worksheet Answers worksheet
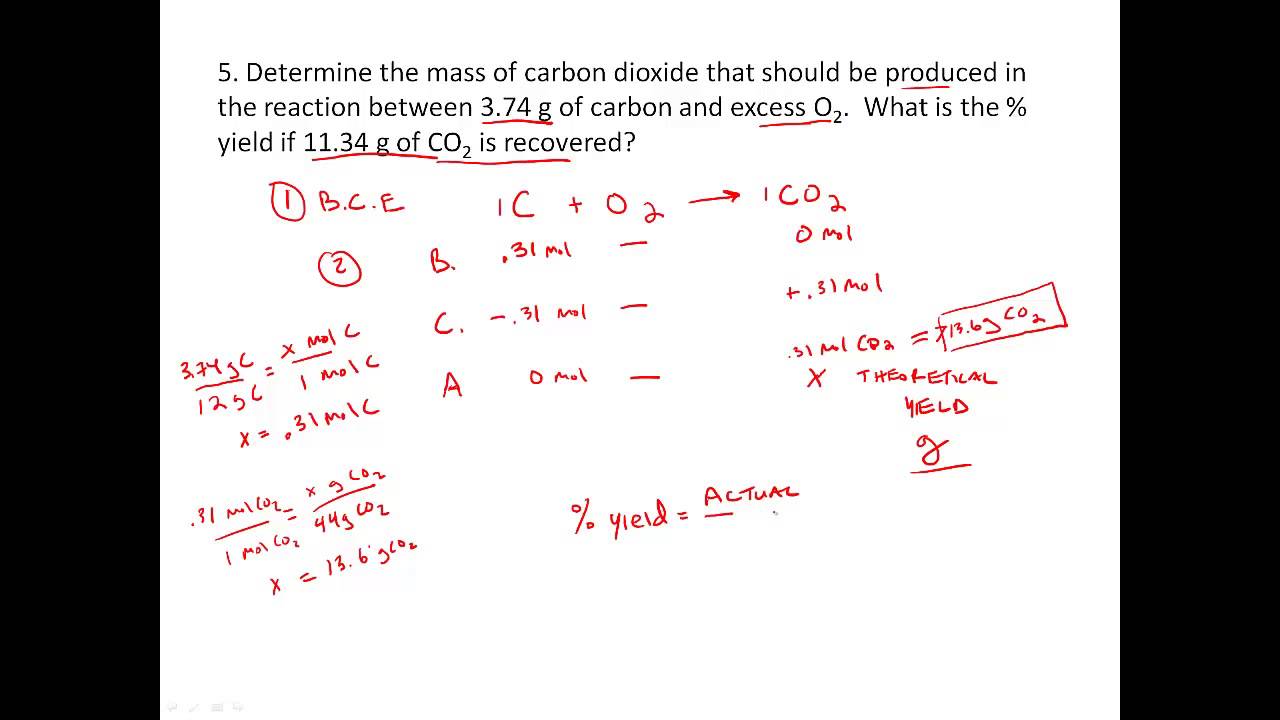
Stoichiometry practice Percent Yield problems YouTube
Molar Mass Worksheet Answer Key
Theoretical and Percent Yield Worksheet Answers
Otis Worksheet List Of Limiting Reactant And Percent Yield Worksheet
Calculate The Percent Yield Of A Reaction That Had A Theoretical Yield Of 3.76 G And An Actual Yield Of 1.45 G.
Etical Yield In Grams Of Co.
4Fes(S)+ 7O (G)→ 2Fe2O3 (S)+ 4So2 (G) Mfes = 87.91 G/Mol Mfe2O3 = 159.69 Nfes = 16.1 G 87.91 G/Mol.
Web This Worksheet Provides Ten Examples For Students To Work Through The Processes Of Determining The Limiting Reactant, Theoretical Yield, And/Or The Percent Yield Of A.
Related Post: