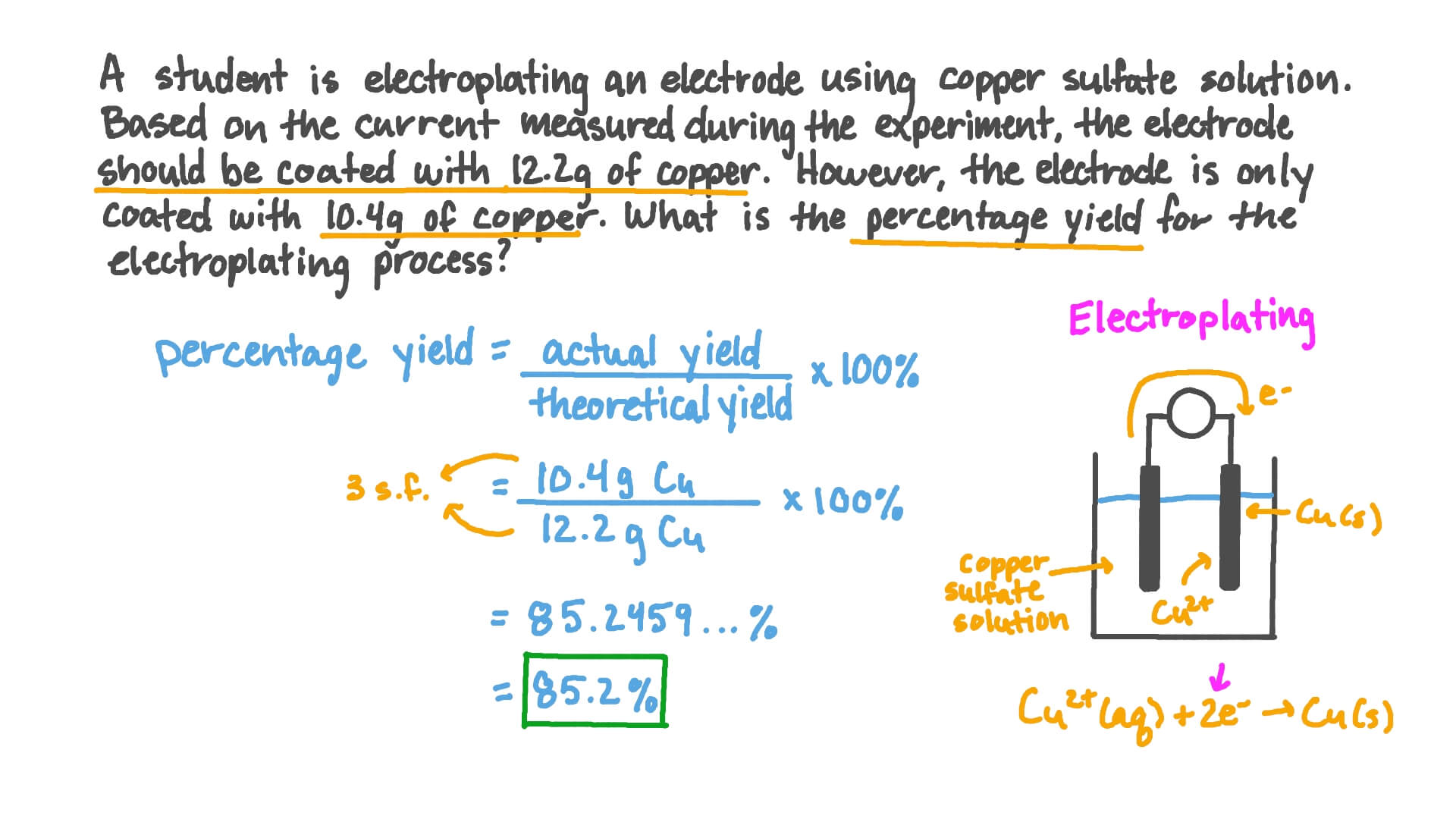
Percent Yield Worksheet
Percent Yield Worksheet - Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. Is heated it decomposes according to the equation: Web up to 24% cash back 1 limiting reactant and percent yield practice name________________________________________ 1) consider the following reaction:. 3 2 kcl + 3 o. Percentage yield practice means progress boost your grades with free daily practice questions. 2) if i perform this. Some of the worksheets for this concept are percent yield work, chemistry percent yield, work percent yield. B) give the % yield if 1.78 g of o. 1) write the equation for the reaction of iron (iii) phosphate with sodium sulfate to make iron (iii) sulfate and sodium phosphate. Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. Web the percent yield of a reaction is the ratio of the actual yield to the theoretical yield, multiplied by 100 to give a percentage: Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. B) give the % yield if 1.78 g of o. Percent yield. Is heated it decomposes according to the equation: Grams of sodium nitrate, how much. B) give the % yield if 1.78 g of o. Percent yield is used in chemistry to evaluate how successful a. Web learn about the percent yield of chemical reactions. The maximum amount of product, which is calculated using the balanced equation actual yield: 1) write the equation for the reaction of iron (iii) phosphate with sodium sulfate to make iron (iii) sulfate and sodium phosphate. Is heated it decomposes according to the equation: Worksheets are percent yield work, work percent yield name, percent yield and limiting reagents, chem1001 work. The amount of product obtained. The practice problems will address finding the percent yield from a single reactant, from two reactants considering the. Is heated it decomposes according to the equation: Web worksheet 12 percent yield in chemical reactions calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45. When carbon disulfide burns in the. Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. If the actual yield is 63.7 g of chlorobenzene, calculate the percent yield. A) calculate the theoretical yield of oxygen. Yield is 72.7 g fes, actual yield is 50.8 g fes. Some of the worksheets for this concept are percent yield work, work percent yield name, percent yield and. Web learn about the percent yield of chemical reactions. What is the theoretical yield if 45.6 g of benzene react? (actual yield / theoretical yield) x 100 = percent yield. 2) if i perform this. (actual yield / theoretical yield) x 100 = percent yield. 3 2 kcl + 3 o. Web the formula for percent yield is: Grams of sodium nitrate, how much. Worksheets are percent yield work, work percent yield name, percent yield and limiting reagents, chem1001 work 5. Is heated it decomposes according to the equation: B) give the % yield if 1.78 g of o. Grams of sodium nitrate, how much. Balance the equation for the reaction given below: The maximum amount of product, which is calculated using the balanced equation actual yield: 3 2 kcl + 3 o. Percent yield is used in chemistry to evaluate how successful a. A) calculate the theoretical yield of oxygen. 1) write the equation for the reaction of iron (iii) phosphate with sodium sulfate to make iron (iii) sulfate and sodium phosphate. Web the formula for percent yield is: Yield is 72.7 g fes, actual yield is 50.8 g fes. 1) write the equation for the reaction of iron (iii) phosphate with sodium sulfate to make iron (iii) sulfate and sodium phosphate. Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. 7) when 5.00 g. Yield is 1.96 g o. The practice problems will address finding the percent yield from a single reactant, from two reactants considering the. 3 2 kcl + 3 o. If the actual yield is 63.7 g of chlorobenzene, calculate the percent yield. Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. 1) write the equation for the reaction of iron (iii) phosphate with sodium sulfate to make iron (iii) sulfate and sodium phosphate. Web if her percent yield was 70.0%, what mass of fes did she get at the end? The maximum amount of product, which is calculated using the balanced equation actual yield: 2) if i perform this. Web up to 24% cash back 1 limiting reactant and percent yield practice name________________________________________ 1) consider the following reaction:. Some of the worksheets for this concept are percent yield work, chemistry percent yield, work percent yield. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. When carbon disulfide burns in the. Yield is 72.7 g fes, actual yield is 50.8 g fes. Cucl2 + nano3 cu(no3)2 + nacl a) if 15 grams of copper (ii) chloride react with 20. What is the theoretical yield if 45.6 g of benzene react? Web up to 24% cash back name chapter 9 percent yield practice worksheet for the balanced equation shown below, if the reaction of 20.7g caco3 produces 6.81g cao, what is the. Percent yield is used in chemistry to evaluate how successful a. Some of the worksheets for this concept are percent yield work, work percent yield name, percent yield and. Percentage yield practice means progress boost your grades with free daily practice questions. Percentage yield practice means progress boost your grades with free daily practice questions. When carbon disulfide burns in the. Web up to 24% cash back name chapter 9 percent yield practice worksheet for the balanced equation shown below, if the reaction of 20.7g caco3 produces 6.81g cao, what is the. Grams of sodium nitrate, how much. The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical. Is heated it decomposes according to the equation: Balance the equation for the reaction given below: (actual yield / theoretical yield) x 100 = percent yield. A) calculate the theoretical yield of oxygen. B) give the % yield if 1.78 g of o. 7) when 5.00 g of kclo. Web the percent yield of a reaction is the ratio of the actual yield to the theoretical yield, multiplied by 100 to give a percentage: The amount of product obtained. Web february 17, 2015 percent yield theoretical yield: What is the theoretical yield if 45.6 g of benzene react? Yield is 72.7 g fes, actual yield is 50.8 g fes.Percent Yield Calculations Worksheet Worksheets For Kindergarten
Percent Yield Worksheet Sulfate Chemical Reactions
SCH 3U Limiting Reagents and Percent Yield Worksheet Given the
30++ Percent Yield Worksheet Answers
Percent Yield Worksheet
20++ Percent Yield Worksheet Worksheets Decoomo
14 Stoichiometry Worksheet 2 Answer Key /
Stoichiometry Worksheet 2 Percent Yield Answers Kayra Excel
Pemdas Worksheets with Answers
Percent Yield of Sodium Carbonate Worksheet Written by A. Norick 39
Yield Is 1.96 G O.
The Maximum Amount Of Product, Which Is Calculated Using The Balanced Equation Actual Yield:
The Practice Problems Will Address Finding The Percent Yield From A Single Reactant, From Two Reactants Considering The.
Web If Her Percent Yield Was 70.0%, What Mass Of Fes Did She Get At The End?
Related Post: