Periodic Trends Worksheet Answer Key
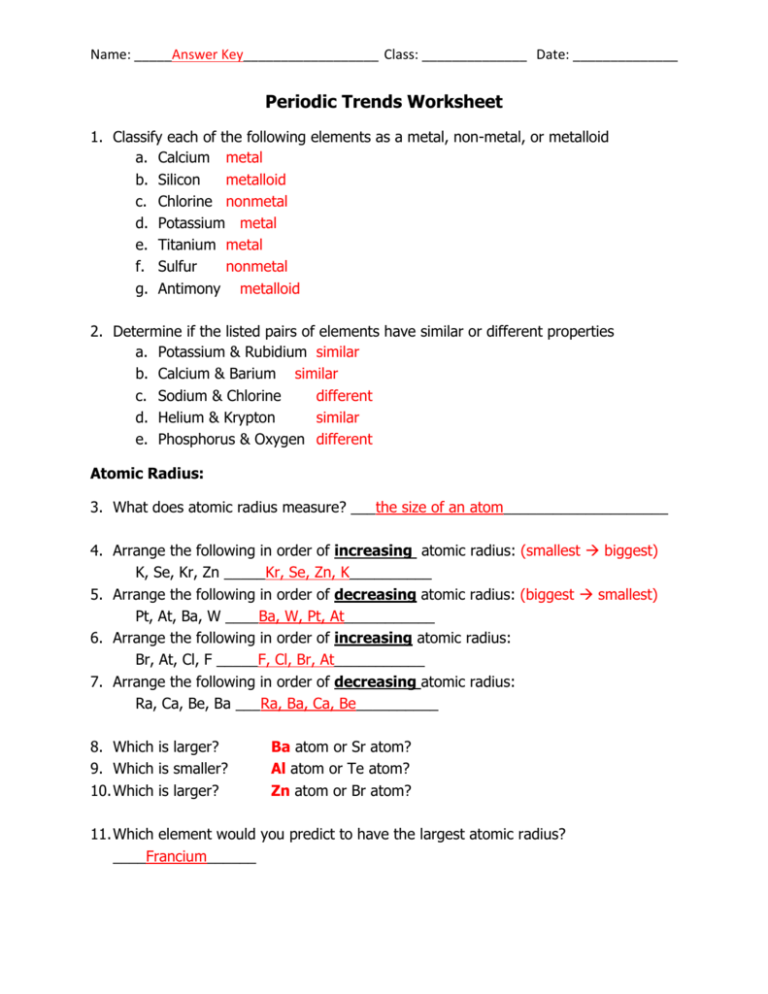
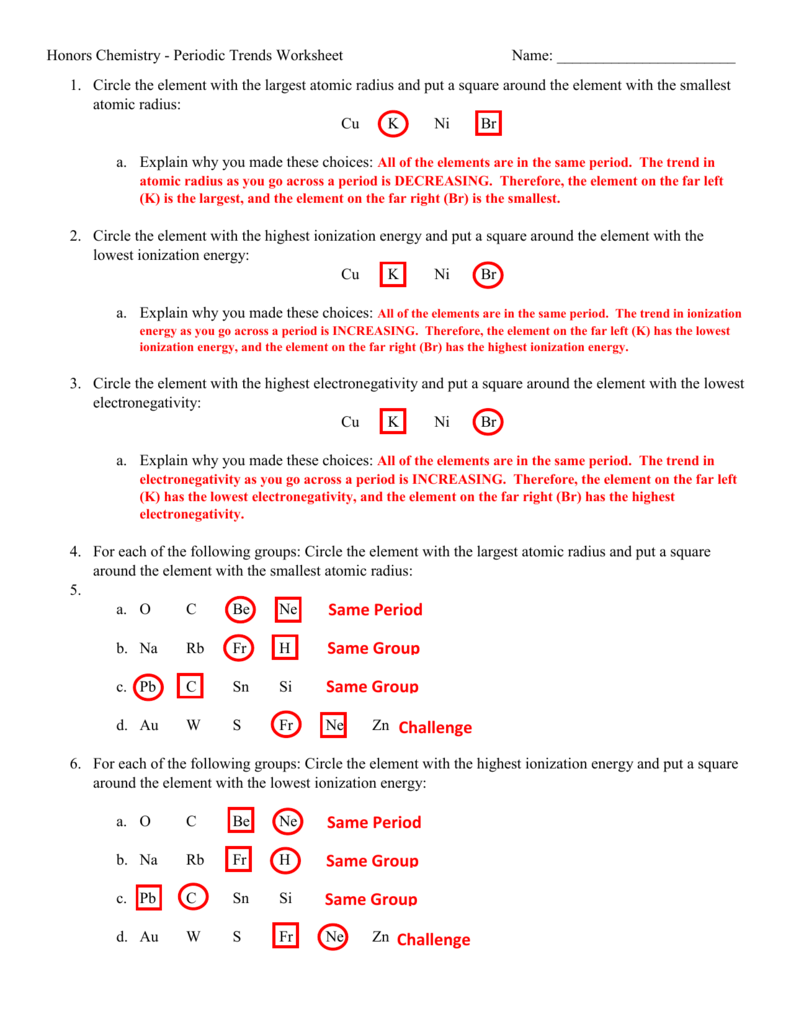
Periodic Trends Worksheet Answer Key - Circle the atom in each pair that has the largest atomic radius. Rank the following elements by increasing electronegativity: Add to my workbooks (16) download file pdf embed in my website or blog add to google classroom Hence the energy required to do so (ionization energy) increases. Visual summary of periodic trends figure 1. All of the elements are in the same period. Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. What trends do you notice for the atomic radii of group 2a? So it becomes harder to remove electron from the atom. Li is the largest because it has the smallest nuclear charge and pulls the electrons toward the nucleus less than the others. To print or download this file, click the link below: Draw a picture to support a written definition of the word “radius.” Web periodic trends answer key. Advanced periodic trends pogil worksheet here. Hence the energy required to do so (ionization energy) increases. *click on open button to open and print to worksheet. A) li or k b) ca or ni c) ga or b d) o or c e) cl or f) be or ba g) si or s h) fe or br au c ) ga or b 2. Atomic number for group 2a and for period 3 of the periodic. Web ionization energy increases from left to right across the period. Which atom in each pair has the larger atomic radius? Circle the atom in each pair that has the largest atomic radius. Using the data below, make a bar graph of atomic radius vs. You will be asked to interact with select atoms as you investigate these concepts. What trends do you notice for the atomic radii of group 2a? Hence the energy required to do so (ionization energy) increases. Arrows to show increasing periodic trends or decreasing periodic trends. Which atom in each pair has the larger atomic radius? What is the periodic trend for atomic size from top to bottom in a group? Hence the energy required to do so (ionization energy) increases. Visual summary of periodic trends figure 1. Calculation of kc and kp. Web know periodic trends of atomic size, ionic size, ionization energy, and electron affinity. Web periodic trends answer key. Circle the atom in each pair that has the largest atomic radius. Periodic trends the periodic table is useful for predicting behavior of elements if you know periodic trends in. The atomic size becomes smaller from left to right. Li, c, f all are in the same period and thus have the same number of energy levels. Mg , si. Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. Web terms in this set (14) rank the following elements by increasing atomic radius: In this investigation you will examine several periodic trends, including atomic radius, ionization energy and ionic radius. Visual summary of periodic trends figure 1. Rank the following. Al or b na or al Calculation of kc and kp answer key; *click on open button to open and print to worksheet. Web periodic trends answer key. Potassium is in the far left group of period 4, and bromine is the farthest to the right of the four elements. What trends do you notice for the atomic radii of group 2a? Rank the following elements by increasing electronegativity: Web ionization energy increases from left to right across the period. Web know periodic trends of atomic size, ionic size, ionization energy, and electron affinity. All of the elements are in the same period. What trends do you notice for the atomic radii of group 2a? To print or download this file, click the link below: Web here are answers for the questions above. Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. Rank the following elements by increasing electronegativity: Atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. A) li or k b) ca or ni c) ga or b d) o or c e) cl or f) be or ba g) si or s h) fe or br au c ) ga or b 2. Li, c, f all are in the same period and thus have the same number of energy levels. Explain why this trend occurs. Rank the following elements by increasing atomic radius: Ionization energy is the amount of energy required to remove an electron from an element. Rank the following elements by increasing electronegativity: Web what is the periodic trend for atomic size from top to bottom in a group? Atomic number for group 2a and for period 3 of the periodic table. What trends do you notice for the atomic radii of group 2a? To print or download this file, click the link below: Web a summary worksheet activity of the periodic table trends including: Visual summary of periodic trends figure 1. Calculation of kc and kp answer key; What trends do you notice for the atomic radii of period 3? Increases due to adding ener gy levels left to right: Hence the energy required to do so (ionization energy) increases. Calculation of kc and kp. Al or b na or al Normalized probability function of the 1s, 2s, and 2p orbitals illustrates orbital penetration in the first two shells. Cu k ni br a. Al, cl, ga cli ionic radius for each of the following sets of ions, rank them from smallest to largest ionic radius. Mg , si b.mg , ca , ba c. Normalized probability function of the 1s, 2s, and 2p orbitals illustrates orbital penetration in the first two shells. All of the elements are in the same period. Rank the following elements by increasing electronegativity: Atomic radius, electron affinity, electron cloud, energy level, group, ion, ionization energy, metal, nonmetal, nucleus, period, periodic trends, picometer, valence electron Atomic number for group 2a and for period 3 of the periodic table. Al or b na or al Rank the following elements by increasing atomic radius: Which atom in each pair has the larger atomic radius? What trends do you notice for the atomic radii of period 3? Using the data below, make a bar graph of atomic radius vs. Explain why you made these choices: Use your notes to answer the following questions. Increases due to adding ener gy levels left to right:Periodic Trends Worksheet Answer Key Homemadeal
periodic trends worksheet with answers
Worksheet Periodic Trends Answers Unique Periodic Trends Worksheets
Periodic Trends Worksheet Answers
Periodic Table Information Worksheet Answers
8 Pics Trends Of The Periodic Table Worksheet Part 1 Answer Key And
Periodic Trends Practice Worksheet
Periodic Trends Practice Worksheet
Periodic Table Worksheet Answer Key Pdf Periodic Table Worksheet
Periodic Trends Worksheet Answer Key
In This Investigation You Will Examine Several Periodic Trends, Including Atomic Radius, Ionization Energy And Ionic Radius.
Web Periodic Trends Answer Key.
Periodic Trends The Periodic Table Is Useful For Predicting Behavior Of Elements If You Know Periodic Trends In.
Draw A Picture To Support A Written Definition Of The Word “Radius.”
Related Post: