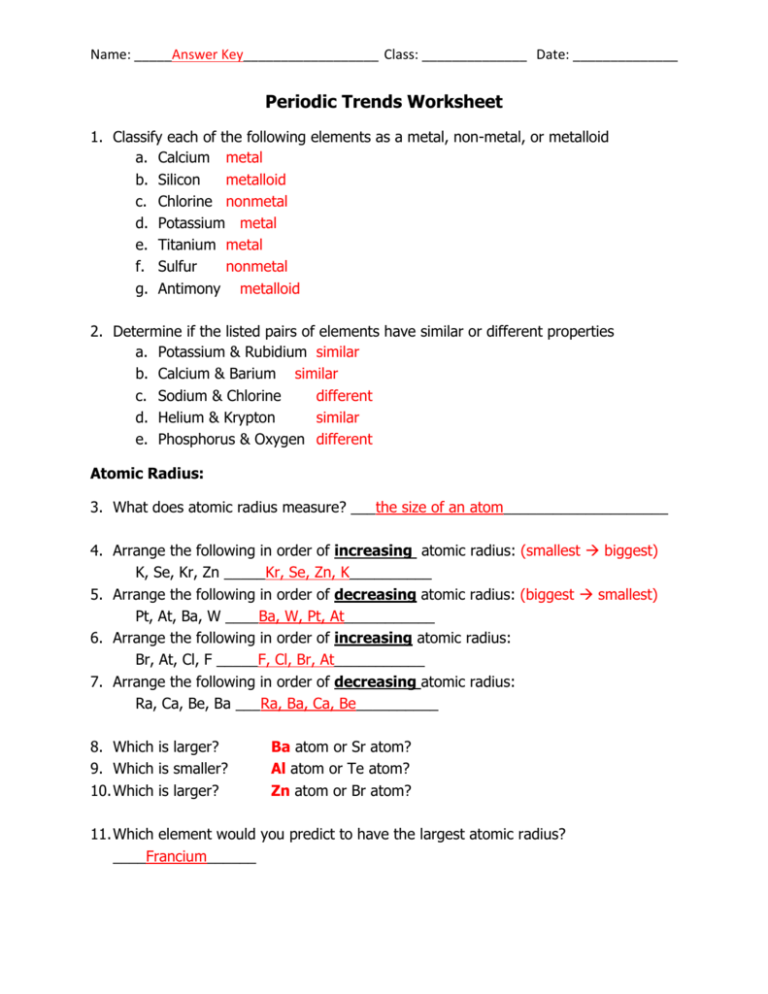
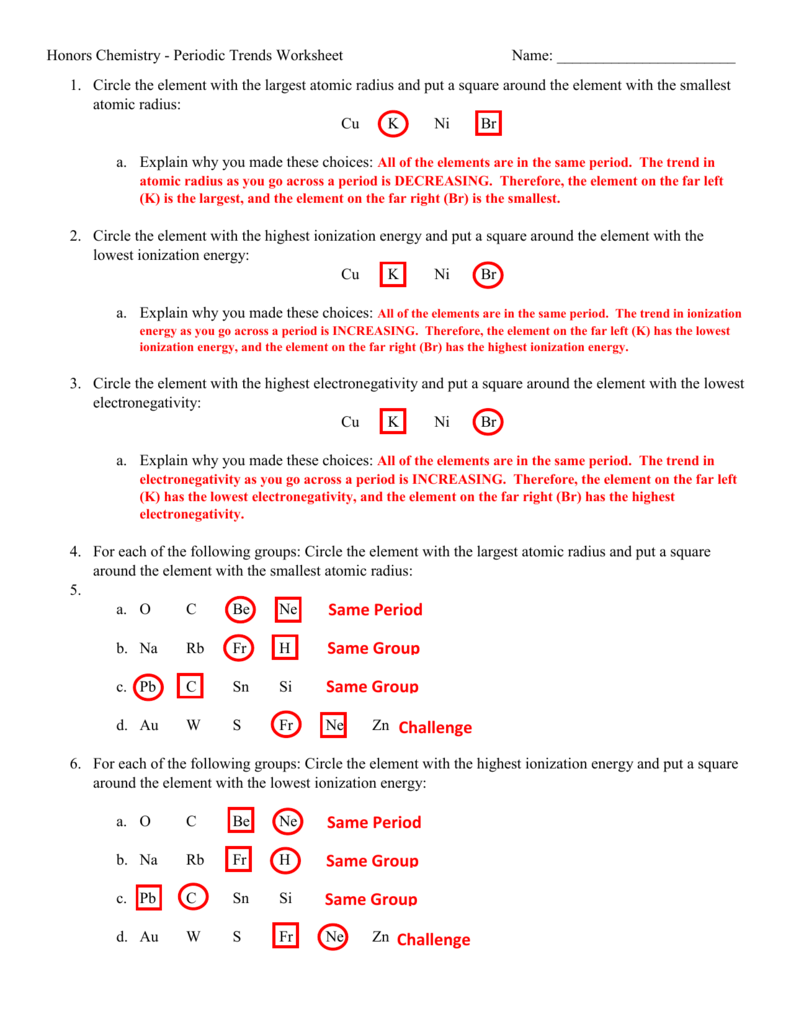
Periodic Trends Worksheet
Periodic Trends Worksheet - Web periodic trends worksheet directions: Neon < aluminum < sulfur. Rank the following elements by increasing electronegativity: \pu {2672 kj/mol} 2672 kj/mol, because the electron removed during the third ionization is a core electron. Worksheets are periodic trends work, name work periodic. Periodic trends (worksheet) learning objective. Use your notes to answer the following questions. What trend in atomic radius do you see as you go across a period/row on the periodic table? What trend in atomic radius do you see as you go down a group/family on the periodic table? Web periodic trends last updated sep 14, 2022 periodic properties of the elements periodic trends in ionic radii periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element, including its size and its electronic properties. The worksheet also explains the various properties that are determined by the periodic trends of elements. What is the order of penetration of the orbitals shown in figure 2? Arrows to show increasing periodic trends or decreasing periodic trends. Revision 3 classification of elements and periodicity in properties. Carbon, aluminum, oxygen, potassium click the card to flip 👆 oxygen, carbon,. \pu {2672 kj/mol} 2672 kj/mol, because the electron removed during the third ionization is a core electron. Neon < aluminum < sulfur. What is the order of penetration of the orbitals shown in figure 2? Use your notes to answer the following questions. Periodic table trends video notes. Carbon, aluminum, oxygen, potassium click the card to flip 👆 oxygen, carbon, aluminum, potassium click the card to flip 👆 1 / 14 flashcards learn test match created by cnners16 terms in this set (14) How does this effect their relative energies? Li, c, f li, na, k ge, p, o c, n, al al, cl, ga ionic radius for. Rank the following elements by increasing electronegativity: \pu {2672 kj/mol} 2672 kj/mol, because the electron removed during the third ionization is a core electron. Li, c, f li, na, k ge, p, o c, n, al al, cl, ga ionic radius for each of the following sets of ions, rank them from smallest to largest ionic radius. How does this. Use your notes to answer the following questions. \pu {2672 kj/mol} 2672 kj/mol, because the electron removed during the third ionization is a core electron. Circle the atom in each pair that has the largest atomic radius. Revision 3 classification of elements and periodicity in properties. Periodic trends atomic radius for each of the following sets of atoms, rank the. The periodic trends chemistry worksheet is a document that explains the chemical trends of elements in a periodic table. Web a summary worksheet activity of the periodic table trends including: Describe the contributions made by the following scientists: What trend in atomic radius do you see as you go down a group/family on the periodic table? Rank the following elements. The worksheet also explains the various properties that are determined by the periodic trends of elements. Oxygen < carbon < aluminum < potassium rank the following elements by increasing electronegativity: Rank the following elements by increasing atomic radius: Electronegativity, electron affinity, ionization energy, ionic radius, atomic radius and shielding effect. Use your notes to answer the following questions. What trend in atomic radius do you see as you go across a period/row on the periodic table? Predict which electrons would experience a higher z eff, 3 d or 4 s ? Web periodic trends predict the trends id: Rank the following elements by increasing electronegativity: Web what is a periodic trend chemistry worksheet? Use your notes to answer the following questions. Describe the contributions made by the following scientists: Web periodic trends last updated sep 14, 2022 periodic properties of the elements periodic trends in ionic radii periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element, including its size and its electronic. Web periodic trends worksheet 4.8 (4 reviews) rank the following elements by increasing atomic radius: Web periodic trends predict the trends id: From left to right in a period? \pu {2672 kj/mol} 2672 kj/mol, because the electron removed during the third ionization is a core electron. Web cell types gizmo worksheet. Circle the atom in each pair that has the largest atomic radius. Periodic trends atomic radius for each of the following sets of atoms, rank the atoms from smallest to largest atomic radius. Rank the following elements by increasing atomic radius: Web periodic trends worksheet #1 1. Periodic table trends video notes. The worksheet also explains the various properties that are determined by the periodic trends of elements. Oxygen < carbon < aluminum < potassium rank the following elements by increasing electronegativity: \pu {2672 kj/mol} 2672 kj/mol, because the electron removed during the third ionization is a core electron. Web periodic trends worksheet 4.8 (4 reviews) rank the following elements by increasing atomic radius: Web periodic trends predict the trends id: Rank the following elements by increasing atomic radius: Rank the following elements by increasing electronegativity: Web periodic trends last updated sep 14, 2022 periodic properties of the elements periodic trends in ionic radii periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element, including its size and its electronic properties. What trend in atomic radius do you see as you go down a group/family on the periodic table? Rank the following elements by increasing electronegativity: Web a summary worksheet activity of the periodic table trends including: From left to right in a period? Use your notes to answer the following questions. Li, c, f li, na, k ge, p, o c, n, al al, cl, ga ionic radius for each of the following sets of ions, rank them from smallest to largest ionic radius. Web periodic trends worksheet. Use your notes to answer the following questions. Oxygen < carbon < aluminum < potassium rank the following elements by increasing electronegativity: Web periodic trends atomic radius 1. Li, c, f li, na, k ge, p, o c, n, al al, cl, ga ionic radius for each of the following sets of ions, rank them from smallest to largest ionic radius. What is the order of penetration of the orbitals shown in figure 2? Web periodic trends worksheet. Revision 3 classification of elements and periodicity in properties. Web what is the periodic trend for atomic size from top to bottom in a group? Neon < aluminum < sulfur. Web periodic trends last updated sep 14, 2022 periodic properties of the elements periodic trends in ionic radii periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element, including its size and its electronic properties. Rank the following elements by increasing atomic radius: How does this effect their relative energies? Trends in sizes of atoms. Write the noble gas electron configuration for the following elements: Use your notes to answer the following questions. Web what is a periodic trend chemistry worksheet?Periodic Trends Worksheet Doc technologykafun
Periodic Trends Practice Worksheet
Periodic Trends Worksheet Answers A Brief Periodic Table Trends
Periodic Trends Worksheet Answer Key —
worksheet periodic trends
8 Pics Trends Of The Periodic Table Worksheet Part 1 Answer Key And
Periodic trends worksheet
Periodic Trends Worksheet
Periodic Table trends graphic Teaching chemistry, Chemistry basics
Periodic Trends Worksheet Answers
Be Able To Predict Differences In Ionization Energy And Electron Affinity Among Elements.
The Worksheet Also Explains The Various Properties That Are Determined By The Periodic Trends Of Elements.
Increases Due To Adding Ener Gy Levels Left To Right:
What Trend In Atomic Radius Do You See As You Go Down A Group/Family On The Periodic Table?
Related Post: