Ph And Poh Calculations Worksheet 2
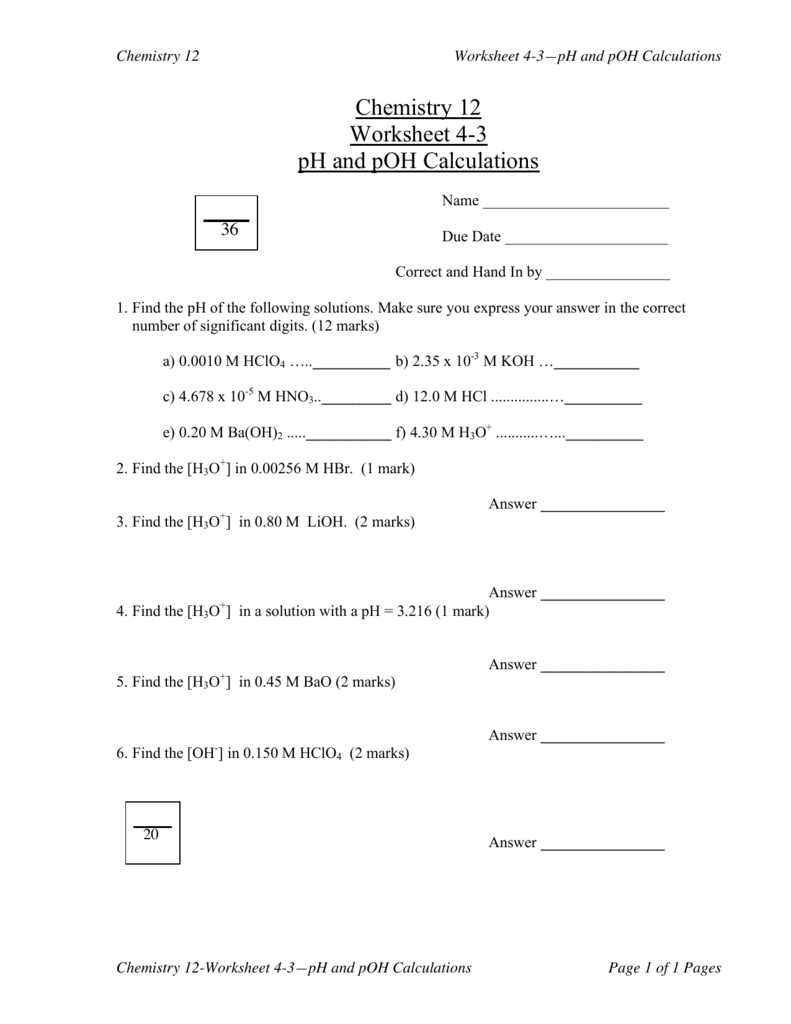
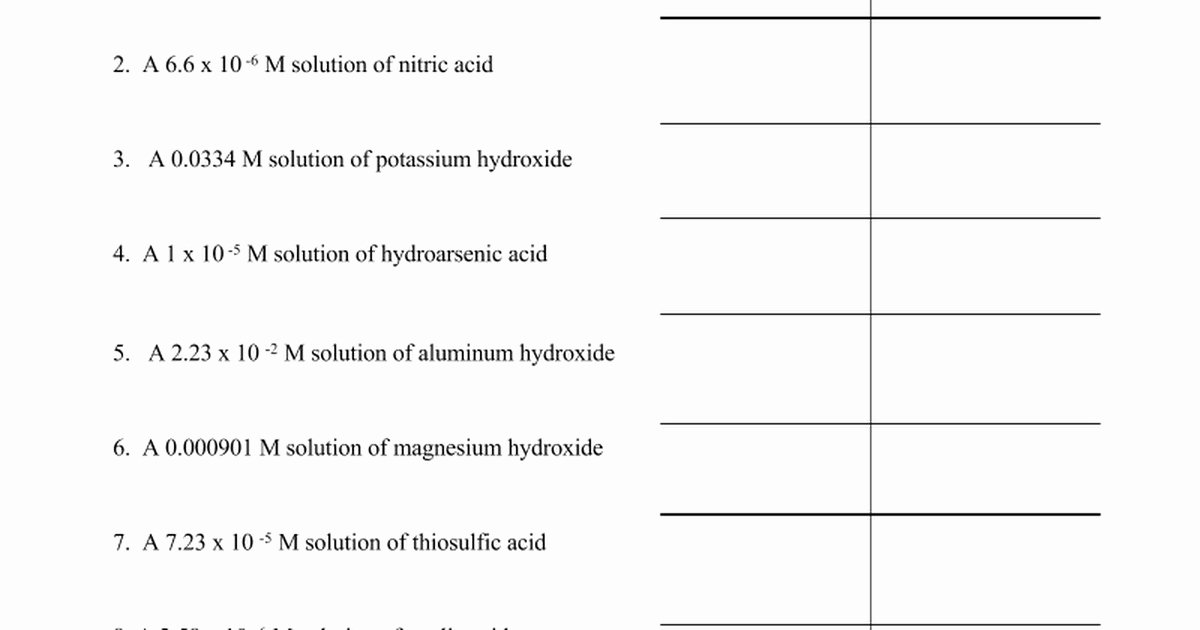
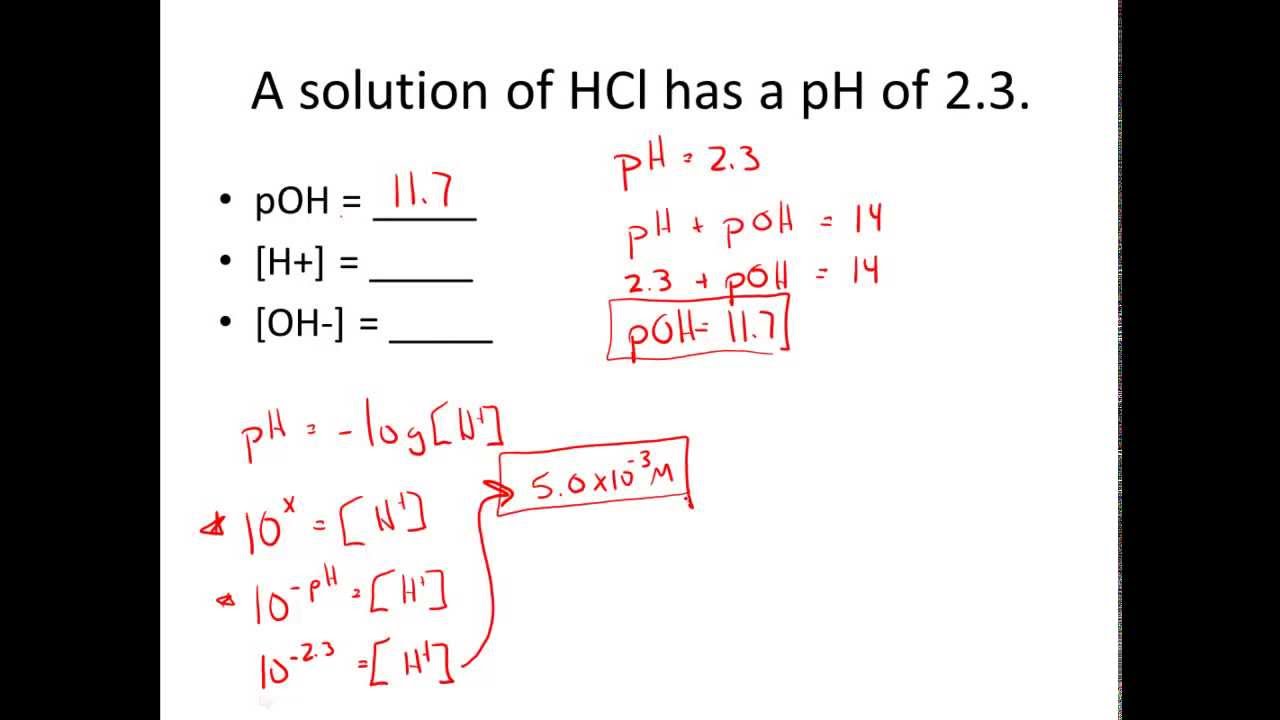
Ph And Poh Calculations Worksheet 2 - Ph = −log[h3o+] = −was log(1.0×10−7) = 7.00 ph = − log [ h 3 o +] = − was log ( 1.0 × 10 − 7) = 7.00 poh=. Web ph and poh calculations worksheet ph and poh calculations worksheet #2 find the ph and the poh of the solution. 2) determine the poh of a 0.00340 m hno3 solution. Ph = −log [ h 3 o +] = −log ( 1.0 × 1 0 −7) = 7.00 poh = −log [ oh −] = −log ( 1.0 × 1 0 −7) = 7.00 Find the ph and the poh of the solution. Fill in the missing information in the table below. A 0.023 m solution of hydrochloric acid 2. Web names _____ date _____ class _____ ph and poh calculations worksheet. Web the ph and poh of a neutral solution at this temperature are therefore: In both print & digital. 2) determine the poh of a 0.00340 m hno3 solution. Ph = −log [ h 3 o +] = −log ( 1.0 × 1 0 −7) = 7.00 poh = −log [ oh −] = −log ( 1.0 × 1 0 −7) = 7.00 Web ph and poh worksheet. [h+] = 4.29 × 10−11 m calculate [h+] in each of. 2) determine the poh of a 0.00340 m hno3 solution. The ph is given by the equation. Web free collection of ph and poh calculation worksheets for students if you want to calculate the ph , you have to take the negative log of the hydronium ion concentration. Calculate the ph by rearranging and plugging in the poh: Since hydrochloric. Poh = 4.95 calculate [oh−] in each of the following. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Show your work and use correct sig. Ph and poh calculations part 1 : The pk a is calculated using the expression: Web names _____ date _____ class _____ ph and poh calculations worksheet. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. The pk a is calculated using the expression: 2) determine the poh of a 0.00340 m hno3 solution. Calculate the ph by rearranging and plugging in the poh: [oh−] = 4.59 × 10−13 m 4. Show your work and use correct sig. A 0.023 m solution of hydrochloric acid 2. The pk a is calculated using the expression: Web the ph and poh of a neutral solution at this temperature are therefore: Ph = −log[h3o+] = −was log(1.0×10−7) = 7.00 ph = − log [ h 3 o +] = − was log ( 1.0 × 10 − 7) = 7.00 poh=. Find the ph and the poh of the solution. [h+] = 4.29 × 10−11 m calculate [h+] in each of the following aqueous solutions: At this temperature, then, neutral solutions. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. Calculate the ph by rearranging and plugging in the poh: Ph = −log[h3o+] = −was log(1.0×10−7) = 7.00 ph = − log [ h 3 o +] = − was log ( 1.0 × 10 − 7) = 7.00 poh=. Show your work and use correct sig.. Web ph and poh calculations worksheet ph and poh calculations worksheet #2 find the ph and the poh of the solution. Web browse ph, poh calculation resources on teachers pay teachers, a marketplace trusted by millions of teachers for original educational resources. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. Web up to 6% cash. Web ph and poh worksheet. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. A 0.023 m solution of hydrochloric acid 2. The ph is given by the equation. Web up to 6% cash back we can then derive the poh from the ph value. Ph = 6.0 determine the ph of each solution of the following bases. [h+] = 4.29 × 10−11 m calculate [h+] in each of the following aqueous solutions: Poh = 4.95 calculate [oh−] in each of the following. Find the ph and the poh of the solution. Web calculate the poh by plugging the [oh −] into the equation. In both print & digital. Web what is the ph of this solution? Find the ph and the poh of the solution. Ph = −log[h3o+] = −was log(1.0×10−7) = 7.00 ph = − log [ h 3 o +] = − was log ( 1.0 × 10 − 7) = 7.00 poh=. 2) determine the poh of a 0.00340 m hno3 solution. Web ph and poh worksheet. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. The pk a is calculated using the expression: Web ph and poh calculations worksheet ph and poh calculations worksheet #2 find the ph and the poh of the solution. Web free collection of ph and poh calculation worksheets for students if you want to calculate the ph , you have to take the negative log of the hydronium ion concentration. Fill in the missing information in the table below. Web the ph and poh of a neutral solution at this temperature are therefore: Web browse ph, poh calculation resources on teachers pay teachers, a marketplace trusted by millions of teachers for original educational resources. Web up to 6% cash back we can then derive the poh from the ph value. A 0.023 m solution of hydrochloric acid 2. The ph is given by the equation. Show your work and use correct sig. Ph = 6.0 determine the ph of each solution of the following bases. [oh−] = 4.59 × 10−13 m 4. Calculate the ph by rearranging and plugging in the poh: Fill in the missing information in the table below. A 0.023 m solution of hydrochloric acid 2. Web names _____ date _____ class _____ ph and poh calculations worksheet. [h+] = 4.29 × 10−11 m calculate [h+] in each of the following aqueous solutions: Web browse ph, poh calculation resources on teachers pay teachers, a marketplace trusted by millions of teachers for original educational resources. In both print & digital. Ph = 6.0 determine the ph of each solution of the following bases. Web calculate the poh by plugging the [oh −] into the equation. [oh−] = 4.59 × 10−13 m 4. Calculate the ph by rearranging and plugging in the poh: Find the ph and the poh of the solution. 2) determine the poh of a 0.00340 m hno3 solution. Web ph and poh worksheet. Show your work and use correct sig. Web the ph and poh of a neutral solution at this temperature are therefore: The pk a is calculated using the expression:Ph And Poh Worksheet Answers
Calculating Ph And Poh Worksheet
Ph And Poh Calculations Worksheet 2
Calculating pH and pOH worksheet 2
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
Ph And Poh Calculations Worksheet 2
Ph poh hesaplama Hakkında
Ph And Poh Calculations Worksheet CALCULATOR GWK
Calculating Ph And Poh Worksheet
Ph And Poh Calculations Worksheet
At This Temperature, Then, Neutral Solutions Exhibit Ph = Poh = 6.31, Acidic.
Web Up To 6% Cash Back We Can Then Derive The Poh From The Ph Value.
Ph = −Log [ H 3 O +] = −Log ( 1.0 × 1 0 −7) = 7.00 Poh = −Log [ Oh −] = −Log ( 1.0 × 1 0 −7) = 7.00
Fill In The Missing Information In The Table Below.
Related Post: