Ph And Poh Calculations Worksheet Answer Key
Ph And Poh Calculations Worksheet Answer Key - For each of the problems below , assume 100% dissociation. Web 14.00 = ph + poh. 100 ml 0.1 m hcl + 200 ml of 0.1 m naoh hcl(s) + naoh(s). [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at 25 °c. Web up to 24% cash back ph and poh calculations find the ph and the poh of the solution ph poh 1. Calculate the ph by rearranging and plugging in the poh: What is the ph of the solution? Web up to 6% cash back possible answers: [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: [h+] = 4.59 × 10−7 m 2. Web up to 6% cash back possible answers: Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Uopnps puy noá dn0jb jnoá ssnosya Web up to 6% cash back possible answers: Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Uopnps puy noá dn0jb jnoá ssnosya 2) determine the poh of a 0.00340 m hno3 solution. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Web calculate the poh by plugging the [oh −] into the equation. [h+] = 4.59 × 10−7 m 2. Ph and poh are the log concentrations of protons and hydroxide ions, respectively. Web 14.00 = ph + poh. 100 ml 0.1 m hcl + 200 ml of 0.1 m naoh hcl(s) + naoh(s). Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? What is the ph of the solution? Web here is a very useful worksheet on calculating the ph and poh of acids. [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: Web up to 24% cash back ph and poh calculations find the ph and the poh of the solution ph poh 1. Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! [h+] = 4.29. [h+] = 4.29 × 10−11 m. Web up to 6% cash back possible answers: Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Calculate the ph by rearranging and plugging in the poh: What is the ph of the solution? For each of the problems below , assume 100% dissociation. Fill in the missing information in the table below. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at 25 °c. Web here is a very useful worksheet on calculating the ph and poh of acids. What is the ph of the solution? Web here is a very useful worksheet on calculating the ph and poh of acids and bases.i have included a log table for you convenience. Ph and poh are the log concentrations of protons and hydroxide ions, respectively. Web calculate the poh by plugging the [oh −] into the equation. As was shown. Web up to 24% cash back ph and poh calculations find the ph and the poh of the solution ph poh 1. Calculate the ph by rearranging and plugging in the poh: Uopnps puy noá dn0jb jnoá ssnosya As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7. Ph and poh are the log concentrations of protons and hydroxide ions, respectively. What is the ph of the solution? Uopnps puy noá dn0jb jnoá ssnosya [h+] = 4.29 × 10−11 m. 100 ml 0.1 m hcl + 200 ml of 0.1 m naoh hcl(s) + naoh(s). Web here is a very useful worksheet on calculating the ph and poh of acids and bases.i have included a log table for you convenience. Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! Fill in the missing information in the table below. For each of the problems below , assume 100% dissociation. Web 14.00 = ph + poh. 100 ml 0.1 m hcl + 200 ml of 0.1 m naoh hcl(s) + naoh(s). Student will also familiarizing themselves with the. 2) determine the poh of a 0.00340 m hno3 solution. Calculate the ph by rearranging and plugging in the poh: [h+] = 4.29 × 10−11 m. Ph and poh are the log concentrations of protons and hydroxide ions, respectively. Web up to 6% cash back possible answers: [oh−] = 4.59 × 10−13 m 4. Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: [h+] = 4.59 × 10−7 m 2. The ph and poh of a neutral solution. Web up to 24% cash back ph and poh calculations find the ph and the poh of the solution ph poh 1. What is the ph of the solution? As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at 25 °c. [h+] = 4.59 × 10−7 m 2. [h+] = 4.29 × 10−11 m. Calculate the ph by rearranging and plugging in the poh: [oh−] = 4.59 × 10−13 m 4. For example the ph of a solution is given by. Student will also familiarizing themselves with the. Uopnps puy noá dn0jb jnoá ssnosya 2) determine the poh of a 0.00340 m hno3 solution. The ph and poh of a neutral solution. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Web here is a very useful worksheet on calculating the ph and poh of acids and bases.i have included a log table for you convenience. [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: For each of the problems below , assume 100% dissociation. Web up to 24% cash back ph and poh calculations find the ph and the poh of the solution ph poh 1. The sum of ph and poh is.Acids & Bases And Ph Worksheet Pdf Answer Key Islero Guide Answer for
30 Ph and Poh Worksheet Education Template
ph and poh practice worksheet
31 Ph Worksheet Answer Key Education Template
pH and pOH Practice Worksheet
Ph And Poh Worksheet Answer Key Askworksheet
Ph And Poh Calculations Worksheet Answer Key CALCULATOR CGW
ph and poh worksheet answers
Ph And Poh Calculations Worksheet 2 Answer Key → Waltery Learning
30 Ph and Poh Worksheet Education Template
Web Up To 6% Cash Back Possible Answers:
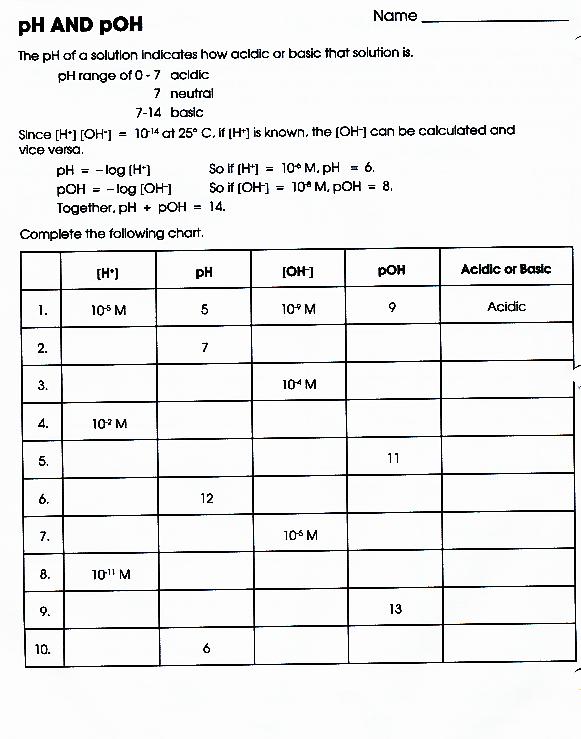
Fill In The Missing Information In The Table Below.
Ph And Poh Are The Log Concentrations Of Protons And Hydroxide Ions, Respectively.
Uot Pp01,N U10Jj Saldurexa Btllsn A.rnpaoo.ld Noåj!
Related Post:





/pHWorksheetAnswers-56a12dd93df78cf772682de3.png)