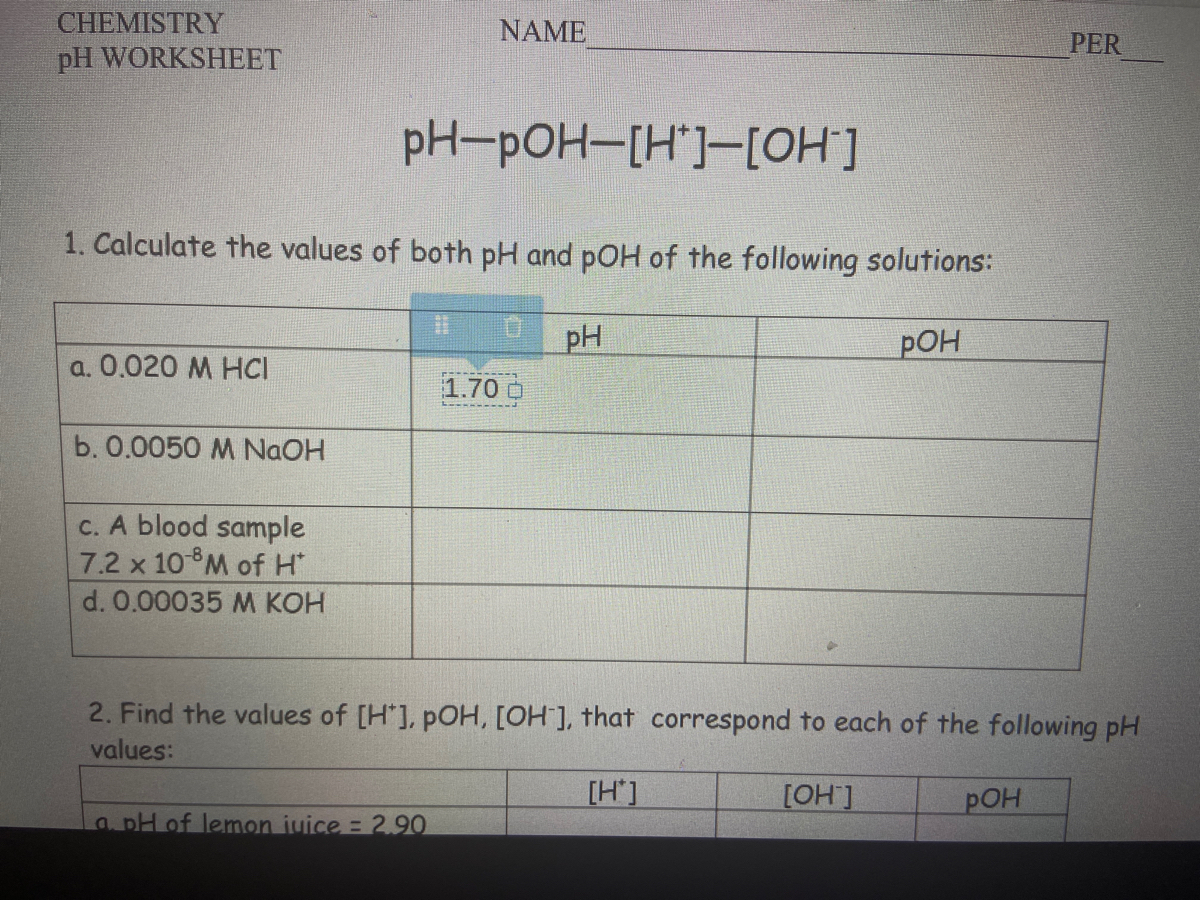
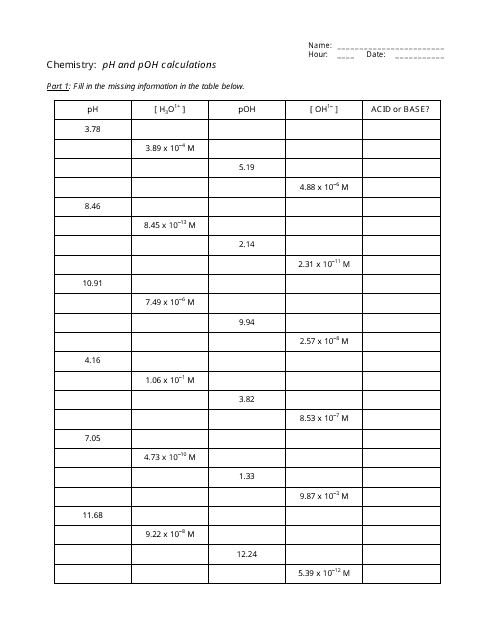
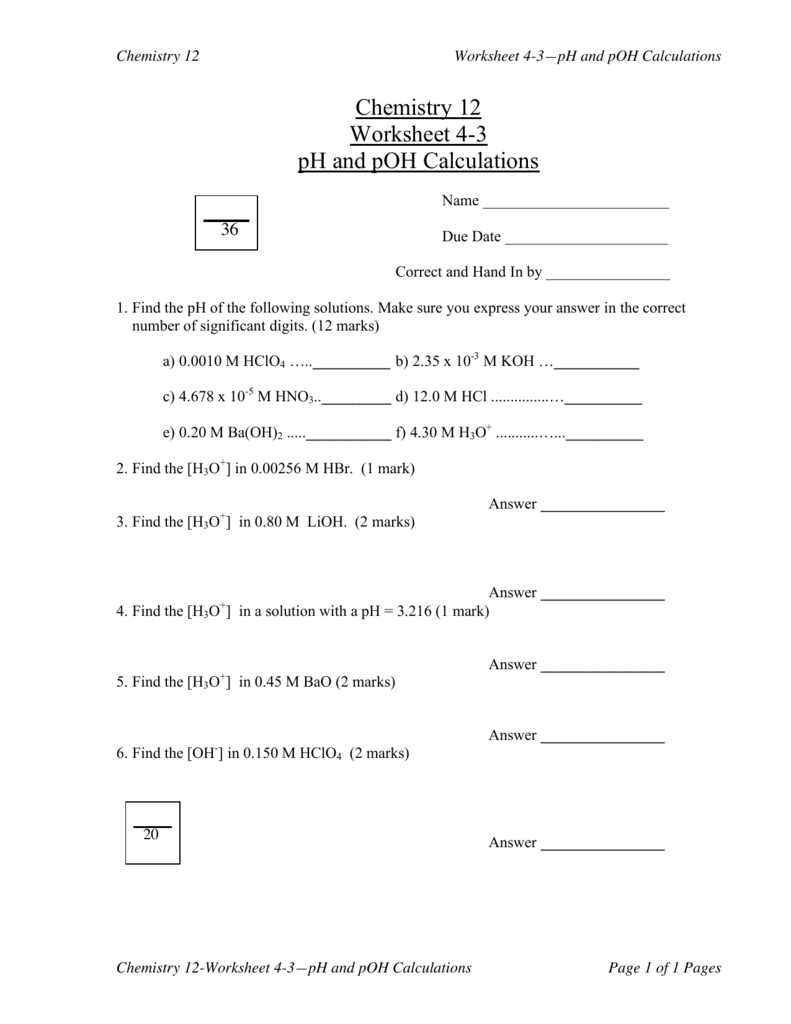
Ph And Poh Calculations Worksheet Answers
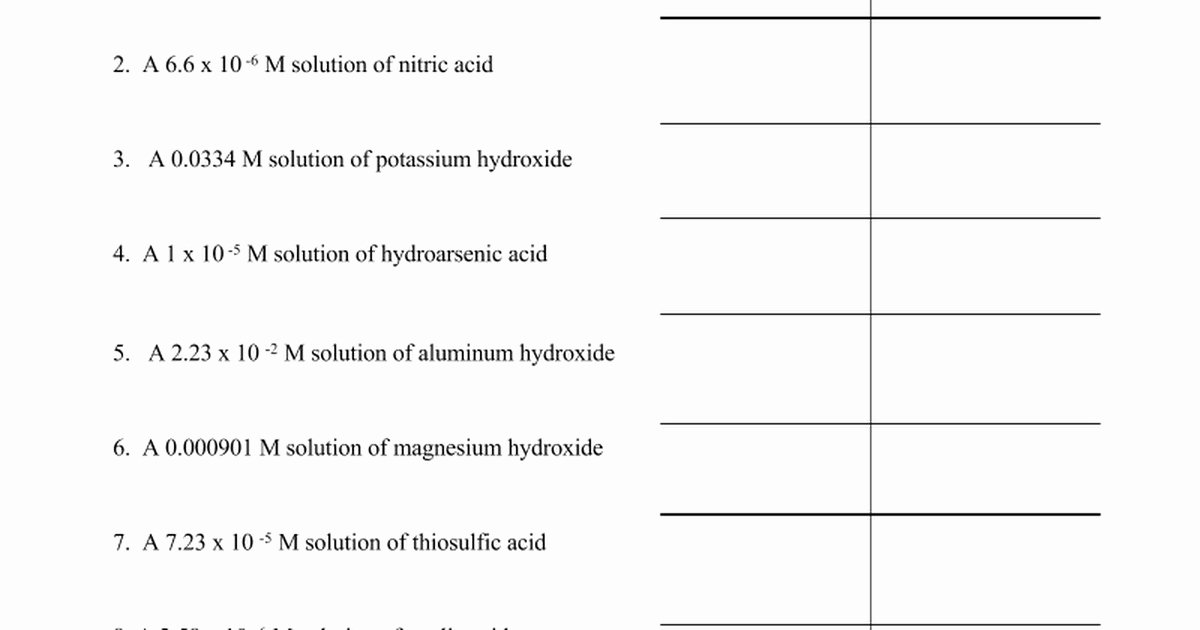
Ph And Poh Calculations Worksheet Answers - [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: Web ph & poh calculations quiz. Web (a) ph = 3.587; Fill in the missing information in the table below. Web find the ph and the poh of the solution ph poh. Using the table of relative strengths of acids and bases to answer the following questions: Web what is the ph of this solution? [h+] = 4.59 × 10−7 m 2. Web 14.00 = ph + poh. Calculate the ph by rearranging and plugging in the poh: Web (a) ph = 3.587; [oh−] = 4.59 × 10−13 m 4. Web practice problems, calculating the ph and poh of strong and weak acids and bases, examples of ph of salt solutions. Web ph and poh calculations part 1 : As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0. H2o, hno3, hocl, nh4 + 2. Web find the ph and the poh of the solution ph poh. First, we'll walk through the possible approaches for calculating [h₃o⁺] from poh. Write a chemical equation, identify the limiting reactant (if there is one), and. Web ph and poh calculations part 1 : 2) determine the poh of a 0.00340 m hno3. Calculate the ph by rearranging and plugging in the poh: Order the following from the strongest to the weakest acid: Web this worksheet is for students to practice calculating ph and poh. Using the table of relative strengths of acids and bases to answer the following questions: 1) what is the ph of a 0.0235 m hcl solution? [h+] = 4.59 × 10−7 m 2. 1) determine the ph of a 0.00340 m hno3 solution. Web (a) ph = 3.587; Web calculate the poh by plugging the [oh −] into the equation. H2o, hno3, hocl, nh4 + 2. Write a chemical equation, identify the limiting reactant (if there is one), and. 1) what is the ph of a 0.0235 m hcl solution? Web what is the ph of this solution? Order the following from the strongest to the weakest acid: [h+] = 4.29 × 10−11 m. [oh−] = 4.59 × 10−13 m 4. Web decimal places in the ph or poh and vice versa. [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: Web up to 6% cash back the question asks us to find the ph of the solution, so we will. The ph and poh of a neutral solution. To do so, we simply subtract the poh from 14. [h+] = 4.59 × 10−7 m 2. Poh = 14.4 what are the ph and poh of a solution of 2.0 m hcl, which. H2o, hno3, hocl, nh4 + 2. The pk a is calculated using the expression: The ph of the solution is. Web practice problems, calculating the ph and poh of strong and weak acids and bases, examples of ph of salt solutions. Web up to 6% cash back the question asks us to find the ph of the solution, so we will need to convert poh to. Calculate the ph by rearranging and plugging in the poh: Web (a) ph = 3.587; Web what is the ph of this solution? As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at 25 °c. [h+] = 4.29 × 10−11 m. Using the table of relative strengths of acids and bases to answer the following questions: To do so, we simply subtract the poh from 14. First, we'll walk through the possible approaches for calculating [h₃o⁺] from poh. [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: Order the following from the strongest to. Web 14.00 = ph + poh. To do so, we simply subtract the poh from 14. [oh−] = 4.59 × 10−13 m 4. [h+] = 4.59 × 10−7 m 2. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at 25 °c. Fill in the missing information in the table below. 1) determine the ph of a 0.00340 m hno3 solution. Web find the ph and the poh of the solution ph poh. H2o, hno3, hocl, nh4 + 2. [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: Web up to 6% cash back the question asks us to find the ph of the solution, so we will need to convert poh to ph. 2) determine the poh of a 0.00340 m hno3. Using the table of relative strengths of acids and bases to answer the following questions: Web what is the ph of this solution? Poh = 14.4 what are the ph and poh of a solution of 2.0 m hcl, which. The pk a is calculated using the expression: Order the following from the strongest to the weakest acid: The ph and poh of a neutral solution. Web practice problems, calculating the ph and poh of strong and weak acids and bases, examples of ph of salt solutions. Write a chemical equation, identify the limiting reactant (if there is one), and. Fill in the missing information in the table below. Web in this video, we'll solve for [h₃o⁺] and ph in two different worked examples. Web calculate the poh by plugging the [oh −] into the equation. Web decimal places in the ph or poh and vice versa. [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: Web (a) ph = 3.587; 1) determine the ph of a 0.00340 m hno3 solution. [h+] = 4.29 × 10−11 m. First, we'll walk through the possible approaches for calculating [h₃o⁺] from poh. The ph and poh of a neutral solution. Web ph & poh calculations quiz. Poh = 14.4 what are the ph and poh of a solution of 2.0 m hcl, which. 1) what is the ph of a 0.0235 m hcl solution? Order the following from the strongest to the weakest acid: The pk a is calculated using the expression: Web this worksheet is for students to practice calculating ph and poh.Worksheet Ph Calculations Answers
Ph And Poh Worksheet Answers
Ph And Poh Worksheet Answer Key Askworksheet
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
pH and pOH Practice Worksheet
34 Ph And Poh Worksheet Answers support worksheet
Ph and Poh Calculations Chemistry Worksheet With Answers Download
Ph And Poh Worksheet Answers
Acids & Bases And Ph Worksheet Pdf Answer Key Islero Guide Answer for
Which Is The Stronger Acid, Hcl Or H2O?
Write A Chemical Equation, Identify The Limiting Reactant (If There Is One), And.
Using The Table Of Relative Strengths Of Acids And Bases To Answer The Following Questions:
[Oh−] = 4.59 × 10−13 M 4.
Related Post:



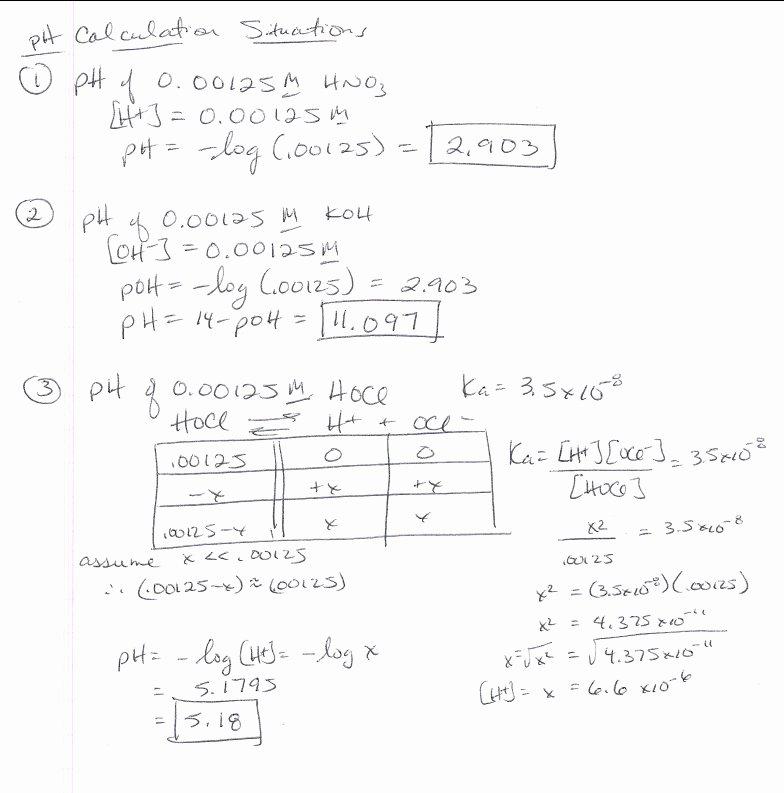
/pHWorksheetAnswers-56a12dd93df78cf772682de3.png)