Ph And Poh Calculations Worksheet
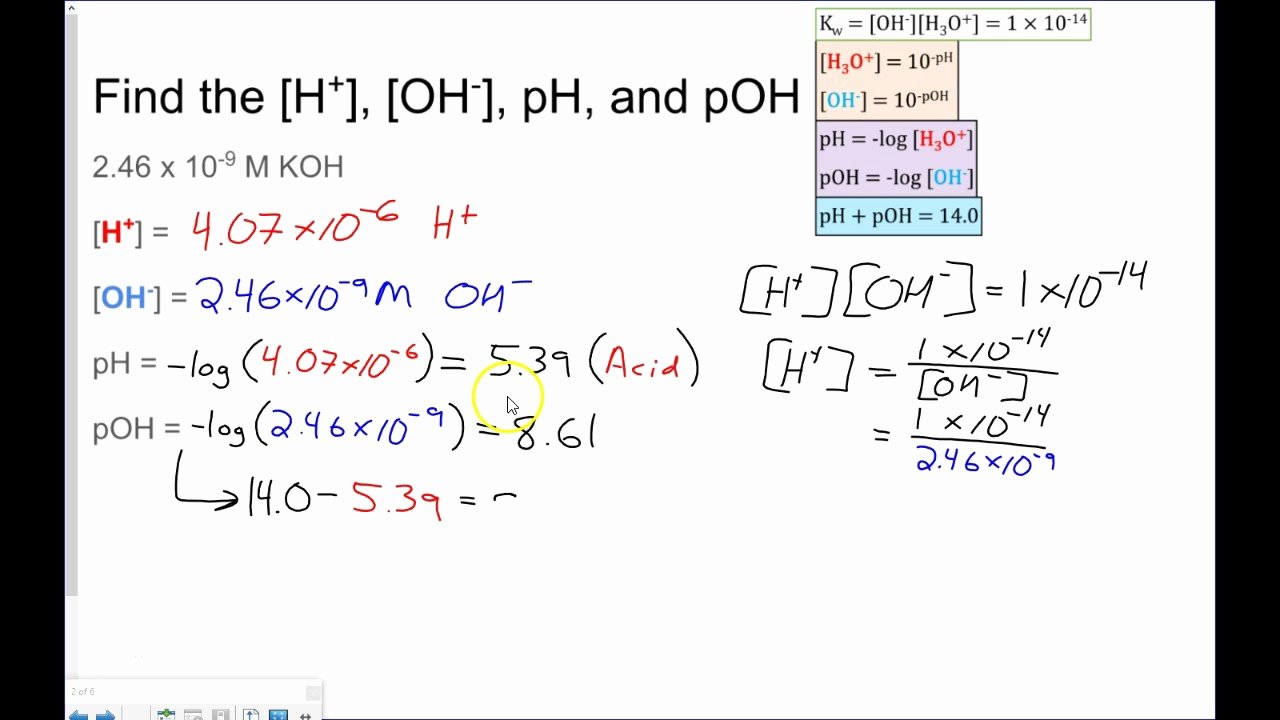
Ph And Poh Calculations Worksheet - Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Web what is the ph of this solution? The only equilibrium concentrations we are concerned with when calculating the ph of a solution. Ph and poh calculations part 1 : Web 0.1 0.1 step 2: If you are struggling with ph and poh calculations , you have come to the right place. First, we'll walk through the possible approaches for calculating [h₃o⁺] from poh. Poh = −log [ oh −] = −log ( 4.9 × 10 −7) = 6.31. Web you find the ph by simply subtracting poh from 14. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. A 0.023 m solution of hydrochloric acid 2. Calculate the ph by rearranging and plugging in the poh: A 0.0334 m solution of potassium hydroxide 4. Ph and poh calculations part 1 : Web concentrations of hydrogen to ph, concentrations of oh to ph all types of ph and poh calculations. The only equilibrium concentrations we are concerned with when calculating the ph of a solution. Web concentrations of hydrogen to ph, concentrations of oh to ph all types of ph and poh calculations. The pk a is calculated using the expression: Includes full solutions and score reporting. Calculate the ph by rearranging and plugging in the poh: 2) determine the poh of a 0.00340 m hno3. All numbers should be rounded to the nearest tenth decimal place.your. Web 0.1 0.1 step 2: Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Web ph = −log [ h 3 o +] = −log ( 4.9 × 10 −7) = 6.31. Web ph = − log[h 3o +] = − log(4.9 × 10 − 7) = 6.31. Fill in the missing information in the table below. All numbers should be rounded to the nearest tenth decimal place.your. Web ph and poh calculations part 1 : Fill in the missing information in the table below. Web ph and poh calculations part 1 : Web in this video, we'll solve for [h₃o⁺] and ph in two different worked examples. The pk a is calculated using the expression: First, we'll walk through the possible approaches for calculating [h₃o⁺] from poh. Poh = −log [ oh −] = −log ( 4.9 × 10 −7) = 6.31. Ph = 6.0 determine the ph of each solution of the following bases. The pk a is calculated using the expression: Web concentrations of hydrogen to ph, concentrations of oh to ph all types of ph and poh calculations. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. What are the equilibrium concentrations of the species. Web calculate the poh by plugging the [oh −] into the equation. Web what is the ph of this solution? 2) determine the poh of a 0.00340 m hno3. Web concentrations of hydrogen to ph, concentrations of oh to ph all types of ph and poh calculations. Poh = −log [ oh −] = −log ( 4.9 × 10 −7). 2) determine the poh of a 0.00340 m hno3. We have collected a myriad of high. Ph and poh calculations part 1 : A 0.023 m solution of hydrochloric acid 2. Web ph, poh, ka & pka worksheet calculate the ph of each of the following aqueous solutions and tell whether the solution is acidic, basic or neutral. The pk a is calculated using the expression: At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. Web what is the ph of this solution? Ph = 6.0 determine the ph of each solution of the following bases. Web ph = −log [ h 3 o +] = −log ( 4.9 × 10 −7) = 6.31. Calculate the ph by rearranging and plugging in the poh: Fill in the missing information in the table below. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. All numbers should be rounded to the nearest tenth decimal place.your. At this temperature, then, neutral solutions exhibit ph = poh =. Web ph, poh, ka & pka worksheet calculate the ph of each of the following aqueous solutions and tell whether the solution is acidic, basic or neutral. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. The only equilibrium concentrations we are concerned with when calculating the ph of a solution. If you are struggling with ph and poh calculations , you have come to the right place. Fill in the missing information in the table below. [h+] = 4.59 × 10−7 m 2. 1) determine the ph of a 0.00340 m hno3 solution. Web ph = − log[h 3o +] = − log(4.9 × 10 − 7) = 6.31. Poh = −log [ oh −] = −log ( 4.9 × 10 −7) = 6.31. First, we'll walk through the possible approaches for calculating [h₃o⁺] from poh. Web ph and poh calculations part 1 : 2) determine the poh of a 0.00340 m hno3. Web you find the ph by simply subtracting poh from 14. Calculate the ph by rearranging and plugging in the poh: We have collected a myriad of high. Fill in the missing information in the table below. Web 0.1 0.1 step 2: Web what is the ph of this solution? A 0.023 m solution of hydrochloric acid 2. A 0.0334 m solution of potassium hydroxide 4. 1) determine the ph of a 0.00340 m hno3 solution. A 0.0334 m solution of potassium hydroxide 4. A 0.023 m solution of hydrochloric acid 2. Web ph and poh calculations part 1 : First, we'll walk through the possible approaches for calculating [h₃o⁺] from poh. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. The only equilibrium concentrations we are concerned with when calculating the ph of a solution. Web ph = − log[h 3o +] = − log(4.9 × 10 − 7) = 6.31. 2) determine the poh of a 0.00340 m hno3. If you are struggling with ph and poh calculations , you have come to the right place. All numbers should be rounded to the nearest tenth decimal place.your. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Fill in the missing information in the table below. Web concentrations of hydrogen to ph, concentrations of oh to ph all types of ph and poh calculations. We have collected a myriad of high. Web 0.1 0.1 step 2:30 Ph and Poh Worksheet Education Template
Ph And Poh Worksheet
50 Ph and Poh Worksheet Chessmuseum Template Library
Ph And Poh Calculations Worksheet —
Calculating Ph and Poh Worksheet With Answers Download Printable PDF
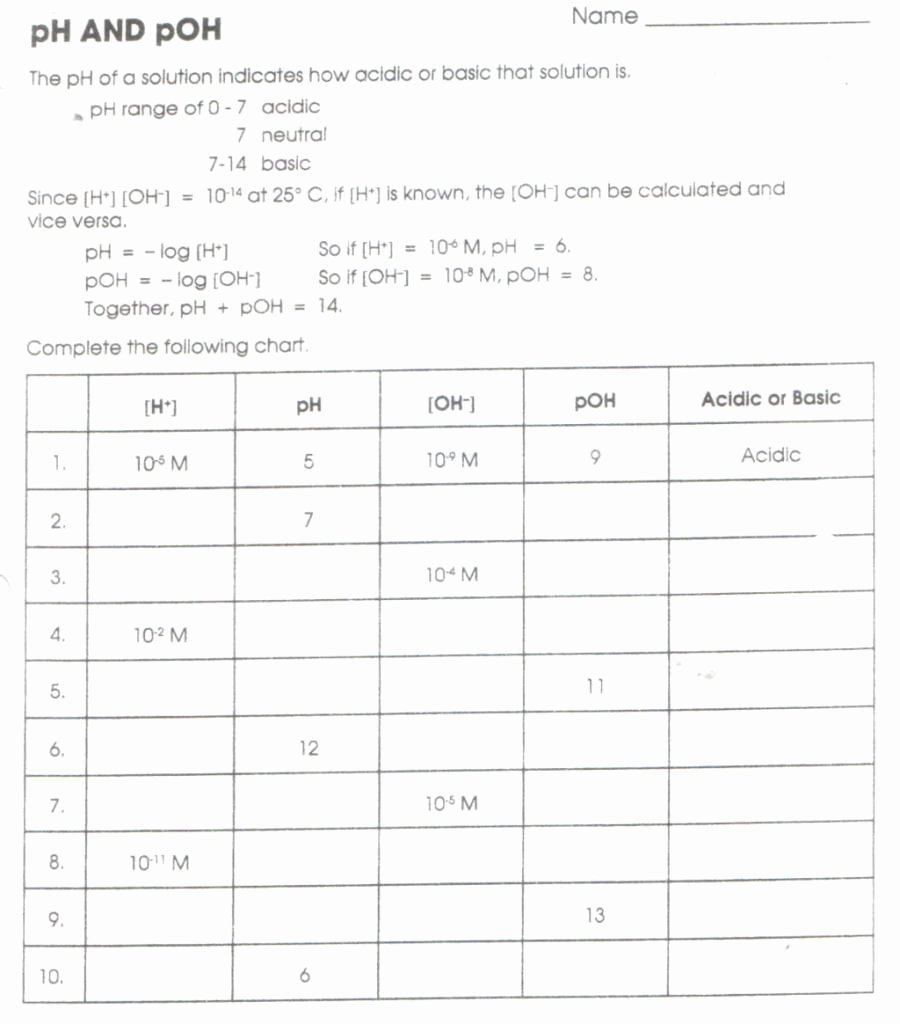
pH and pOH Practice Worksheet
pH and pOH Practice Worksheet
PH and pOH worksheet
50 Ph and Poh Worksheet Chessmuseum Template Library
30 Ph and Poh Worksheet Education Template
Ph = 6.0 Determine The Ph Of Each Solution Of The Following Bases.
Calculate The Ph By Rearranging And Plugging In The Poh:
Web Ph, Poh, Ka & Pka Worksheet Calculate The Ph Of Each Of The Following Aqueous Solutions And Tell Whether The Solution Is Acidic, Basic Or Neutral.
Web In This Video, We'll Solve For [H₃O⁺] And Ph In Two Different Worked Examples.
Related Post:





/pHWorksheet-56a12dd95f9b58b7d0bcd1fc.png)
:max_bytes(150000):strip_icc()/pHWorksheetAnswers-56a12dd93df78cf772682de3.png)