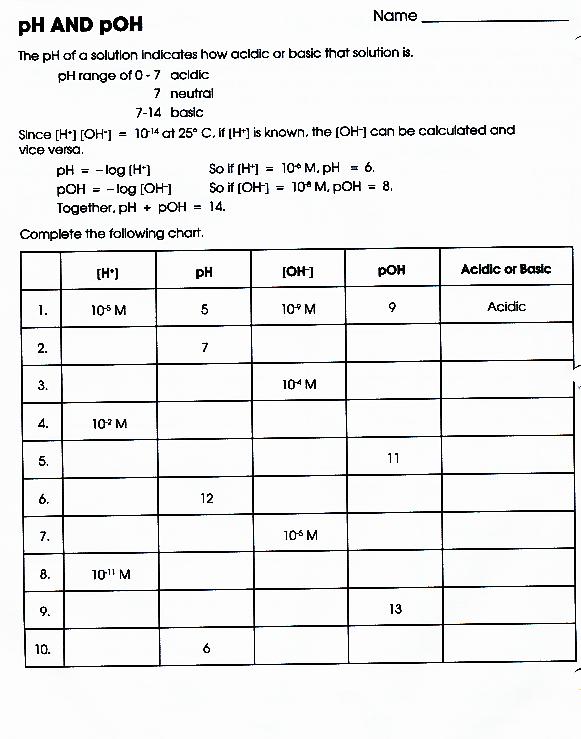
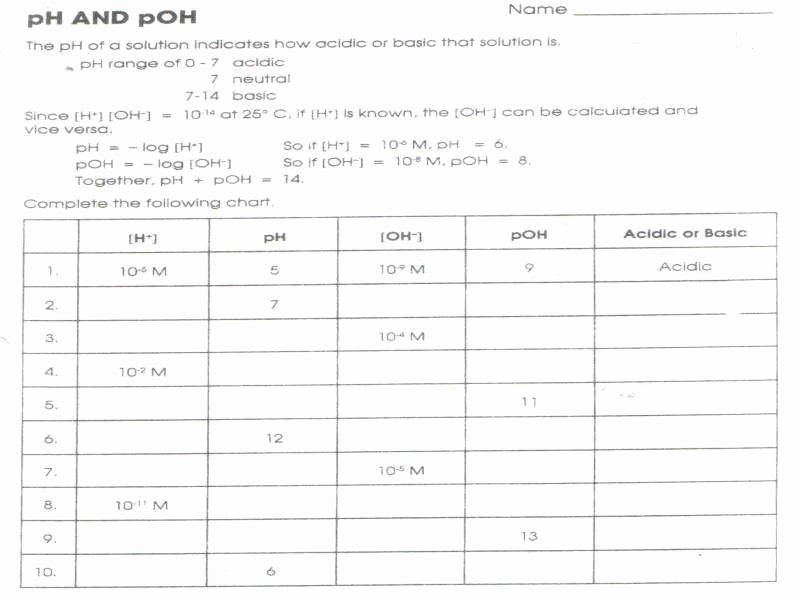
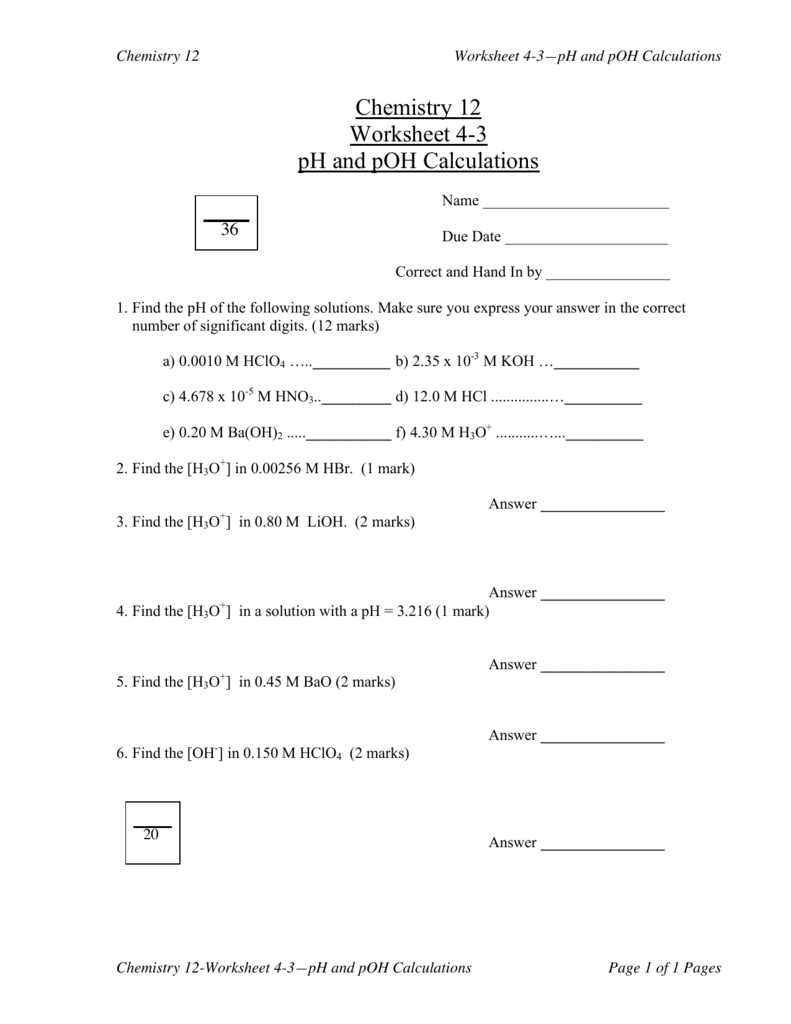
Ph & Poh Worksheet
Ph & Poh Worksheet - A) 0.0010 m hcl ____ g) 0.024 m hcl ____ H2o, hno3, hocl, nh4 + 2. Ph = − log[h3o +] = − log(0.10) = 1.00. Figure 14.2 the ph and poh scales represent concentrations of h3o+ and oh−, respectively. The question asks us to find the ph of the solution, so we will need to convert poh to ph. Web 1) determine the ph of a 0.00340 m hno3 solution. To do so, we simply subtract the poh from 14. [h+] = 4.29 × 10−11 m [oh −] = 0.0044 m × 2 = 0.0088m. The ph and poh values of some common substances at standard temperature (25 °c) are shown in this chart. Because sodium hydroxide is a strong base, it makes sense that the ph is above 7. Chemistry quick review of ph 02 of 02 ph worksheet answers todd. The ph and poh values of some common substances at 25 °c are shown in this chart. [oh −] = 0.0044 m × 2 = 0.0088m. Science quickly review of ph [h+] = 4.29 × 10−11 m Web at this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic solutions exhibit ph less than 6.31 and poh greater than 6.31, whereas basic solutions exhibit ph greater than 6.31 and poh less than 6.31. Because sodium hydroxide is a strong base, it makes sense that the ph is above 7.. [h+] = 4.59 × 10−7 m 2. Web what is ph a measure of? H2o, hno3, hocl, nh4 + 2. A) 0.0010 m hcl ____ g) 0.024 m hcl ____ Order the following from the strongest to the weakest acid: A) 0.0010 m hcl ____ g) 0.024 m hcl ____ Ph and poh calculations part 1: What is the equation that relates to ph and poh? [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: [oh−] = 4.59 × 10−13 m 4. The relationship between acid strength and the ph of a solution. The ph of the solution is 12.3. A) 0.0010 m hcl ____ g) 0.024 m hcl ____ Web at this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic solutions exhibit ph less than 6.31 and poh greater than 6.31, whereas basic solutions exhibit ph greater than. [oh−] = 4.59 × 10−13 m 4. [h+] = 4.59 × 10−7 m 2. What is the equation used for finding ph? Calculate the ph by rearranging and plugging in the poh: The relationship between acid strength and the ph of a solution. The relationship between acid strength and the ph of a solution. The ph and poh scales represent concentrations of [h 3 o +] and oh −, respectively. Web definitions of ph, poh, and the ph scale. Write the equation for the dissociation of hydrochloric acid. Web 2) calculate the ph of solutions having the following ion concentrations at 298k: Science quickly review of ph Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? Poh = − log[oh −] = − log(0.0088) = 2.06. A solution of poh 6.0 or one of poh 8.0. [oh −] = 0.0044 m × 2 = 0.0088m. Worksheets are ph poh h oh work, calculating ph and poh work, work ph calculations name, ph and poh, ph work 2, chemistry 12 ph work epub, ph and poh work answers instructional fair,. Web since we have the concentration of hydroxide ions, we can solve for the poh of the solution. Web definitions of ph, poh, and the ph. Order the following from the strongest to the weakest acid: This distinction can be important when studying certain processes that occur at nonstandard temperatures, such as enzyme. Poh = − log[oh −] = − log(0.0088) = 2.06. Web which solution has a higher ph? For each of the problems below, assume 100% dissociation. A solution of poh 6.0 or one of poh 8.0. [oh −] = 0.0044 m × 2 = 0.0088m. Ph = − log[h3o +] = − log(0.10) = 1.00. Web since we have the concentration of hydroxide ions, we can solve for the poh of the solution. Web ph, poh, ka & pka worksheet calculate the ph of each of the following aqueous solutions and tell whether the solution is acidic, basic or neutral. [oh−] = 4.59 × 10−13 m 4. H2o, hno3, hocl, nh4 + 2. Figure 14.2 the ph and poh scales represent concentrations of h3o+ and oh−, respectively. What is the equation used for finding ph? Web which solution has a higher ph? The ph of the solution is 12.3. Science quickly review of ph Web 2) calculate the ph of solutions having the following ion concentrations at 298k: Poh = − log[oh −] = − log(0.0088) = 2.06. Because sodium hydroxide is a strong base, it makes sense that the ph is above 7. [h+] = 4.29 × 10−11 m The ph and poh values of some common substances at 25 °c are shown in this chart. Calculating the ph of a strong acid or base solution. The ph and poh scales represent concentrations of [h 3 o +] and oh −, respectively. [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: Web what is ph a measure of? Web definitions of ph, poh, and the ph scale. [h+] = 4.29 × 10−11 m [h+] = 4.59 × 10−7 m 2. A) 0.0010 m hcl ____ g) 0.024 m hcl ____ Fill in the missing information in the table below. A 0.1 m solution of an acid with \(k_a = 1 \times 10^{‐4}\) or one with \(k_a = 4 \times 10^{‐5}\) a 0.1 m solution of an acid with \(pk_a = 1.0\) or one with \(pk_a = 3.5\) a 0.1 m solution of a weak acid or a 0.01 m solution of the same acid. Ph = − log[h3o +] = − log(0.10) = 1.00. H2o, hno3, hocl, nh4 + 2. Poh = − log[oh −] = − log(0.0088) = 2.06. What is the equation used for finding ph? Web ph, poh, ka & pka worksheet calculate the ph of each of the following aqueous solutions and tell whether the solution is acidic, basic or neutral. Chemistry quick review of ph 02 of 02 ph worksheet answers todd. Measurement conversions [metric to metric] author: Calculate the ph by rearranging and plugging in the poh: Ph = 14.00 − poh = 14.00 − 2.06 = 11.94.Ph And Poh Calculations Worksheet 2
Ph And Poh Calculations Worksheet CALCULATOR GWK
Assignments SCIENCE WITH SEAFORD
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
Ph And Poh Worksheet Answers
PH and pOH worksheet
30 Ph and Poh Worksheet Education Template
Ph And Poh Calculations Worksheet —
Ph And Poh Calculations Worksheet
Ph And Poh Worksheet Answers
Science Quickly Review Of Ph
[Oh−] = 4.59 × 10−13 M 4.
The Relationship Between Acid Strength And The Ph Of A Solution.
To Do So, We Simply Subtract The Poh From 14.
Related Post: