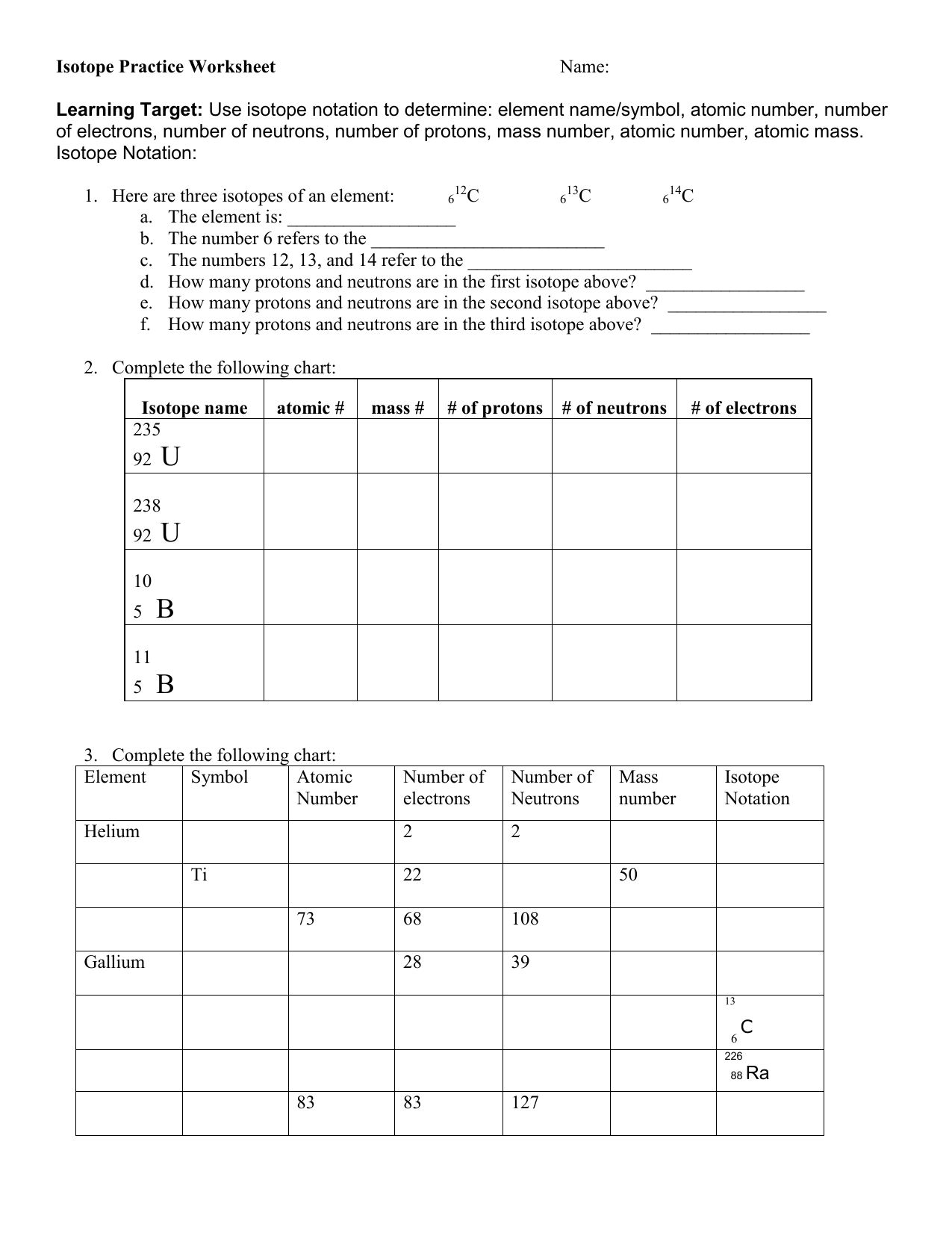
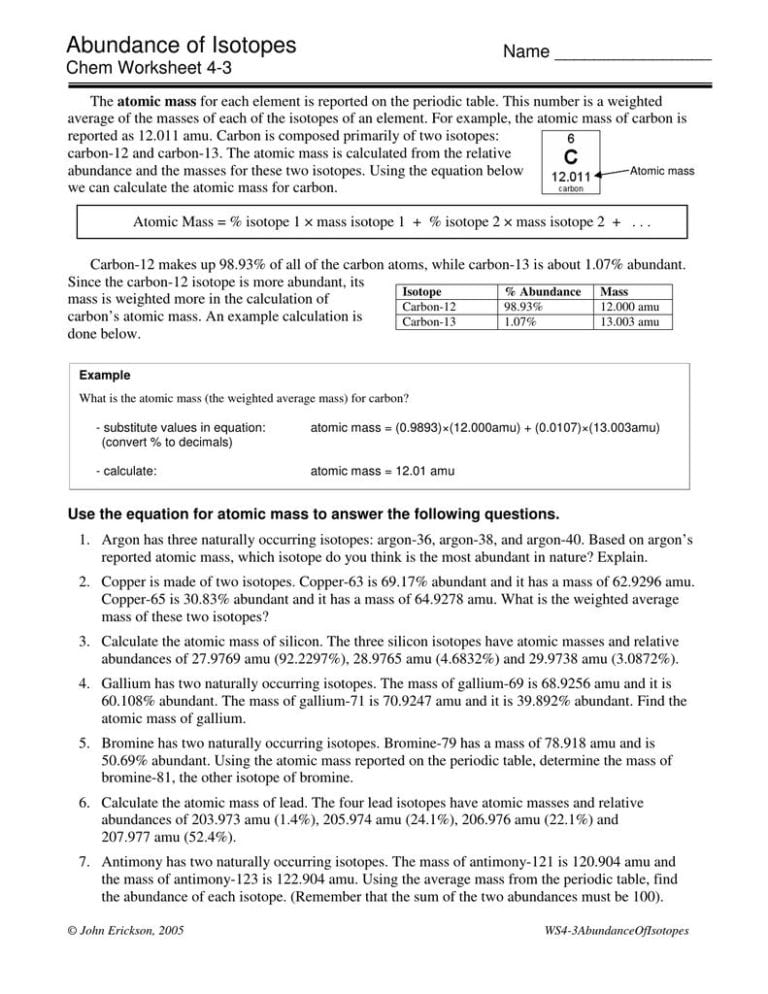
Practice Isotope Calculations 1 Worksheet Answers
Practice Isotope Calculations 1 Worksheet Answers - Web up to 24% cash back fill in the isotope names and any missing information, including isotope numbers from the chart. Free interactive exercises to practice online or download as pdf to print. Web the difference in mass number is due to difference in number of neutrons in the nucleus of the atom. An atom missing or having too many electrons. Some of the worksheets for this concept are isotopesions work element element atomic mass, ,. Web of 78.92 u while 49.46% of the naturally occurring isotopes of bromine have an atomic mass of 80.92 u. A student looked up the naturally occurring isotopes of bromine and found the following information: Calculate the average atomic mass of chlorine if its. Use the periodic table to identify and count subatomic particles within the atom. Add to my workbooks (38) download file pdf embed in. The data of the given isotopes of elements are as follows:. The numbers 12, 13, and 14 refer to the. Web of 78.92 u while 49.46% of the naturally occurring isotopes of bromine have an atomic mass of 80.92 u. 6 12c 6 13c 6 14c a. I can use the atomic number of an element to. Calculate the average atomic mass of chlorine if its. 50.54% of the naturally occurring isotopes of bromine have an atomic mass. A student looked up the naturally occurring isotopes of bromine and found the following information: Use the periodic table to identify and count subatomic particles within the atom. Web the difference in mass number is due to difference in. Web you may be offline or with limited connectivity. The data of the given isotopes of elements are as follows:. An atom missing or having too many electrons. Web an ion is an atom with a non neutral electric charge; Here are three isotopes of an element: I can use the atomic number of an element to. An atom missing or having too many electrons. Calculate the average atomic mass of bromine, showing all work: Web of 78.92 u while 49.46% of the naturally occurring isotopes of bromine have an atomic mass of 80.92 u. The number 6 refers to the atomic number c. Here are three isotopes of an element: Web an ion is an atom with a non neutral electric charge; Use the periodic table to identify and count subatomic particles within the atom. Web the difference in mass number is due to difference in number of neutrons in the nucleus of the atom. Use your periodic table and the information provided. Web of 78.92 u while 49.46% of the naturally occurring isotopes of bromine have an atomic mass of 80.92 u. Some of the worksheets for this concept are isotopesions work element element atomic mass, ,. Web isotope practice worksheet name: Calculate the average atomic mass of chlorine if its. A student looked up the naturally occurring isotopes of bromine and. 6 12c 6 13c 6 14c a. Calculate the average atomic mass of chlorine if its. The data of the given isotopes of elements are as follows:. Some of the worksheets for this concept are isotopesions work element element atomic mass, ,. Use your periodic table and the information provided. Free interactive exercises to practice online or download as pdf to print. Web up to 24% cash back fill in the isotope names and any missing information, including isotope numbers from the chart. The data of the given isotopes of elements are as follows:. Web isotope practice worksheet name: Use the periodic table to identify and count subatomic particles within. Web calculate the atomic weight of an element with two naturally occurring isotopes, from the following data: Web the difference in mass number is due to difference in number of neutrons in the nucleus of the atom. Add to my workbooks (38) download file pdf embed in. Web isotopes worksheets and online activities. Atomic mass of protons of neutrons of. Use the periodic table to identify and count subatomic particles within the atom. 50.54% of the naturally occurring isotopes of bromine have an atomic mass. Calculate the average atomic mass of bromine, showing all work: Some of the worksheets for this concept are isotopesions work element element atomic mass, ,. Here are three isotopes of an element: Atomic mass of protons of neutrons of electrons isotopie symbol (nuclear form)… Add to my workbooks (38) download file pdf embed in. Calculate the average atomic mass of chlorine if its. Web calculate the atomic weight of an element with two naturally occurring isotopes, from the following data: 50.54% of the naturally occurring isotopes of bromine have an atomic mass. I can use the atomic number of an element to. I can use the atomic number of an element to. Calculate the average atomic mass of bromine, showing all work: Web up to 24% cash back fill in the isotope names and any missing information, including isotope numbers from the chart. The numbers 12, 13, and 14 refer to the. Web the difference in mass number is due to difference in number of neutrons in the nucleus of the atom. The number 6 refers to the atomic number c. Web isotope practice worksheet name: Web isotope practice worksheet name: Web isotopes worksheets and online activities. The data of the given isotopes of elements are as follows:. Web an ion is an atom with a non neutral electric charge; A student looked up the naturally occurring isotopes of bromine and found the following information: Web of 78.92 u while 49.46% of the naturally occurring isotopes of bromine have an atomic mass of 80.92 u. Use the periodic table to identify and count subatomic particles within the atom. I can use the atomic number of an element to. Free interactive exercises to practice online or download as pdf to print. Web up to 24% cash back fill in the isotope names and any missing information, including isotope numbers from the chart. Add to my workbooks (38) download file pdf embed in. The data of the given isotopes of elements are as follows:. Some of the worksheets for this concept are isotopesions work element element atomic mass, ,. Web the difference in mass number is due to difference in number of neutrons in the nucleus of the atom. Calculate the average atomic mass of chlorine if its. Use the periodic table to identify and count subatomic particles within the atom. The number 6 refers to the atomic number c. Use the periodic table to identify and count subatomic particles within the atom. A student looked up the naturally occurring isotopes of bromine and found the following information: Web you may be offline or with limited connectivity. Web of 78.92 u while 49.46% of the naturally occurring isotopes of bromine have an atomic mass of 80.92 u. Web isotope practice worksheet name: All atoms are isotopes, regardless of whether or not they are.30 isotope Practice Worksheet Answers Education Template
30 isotope Practice Worksheet Answers Education Template
practice isotope calculations 1 worksheet answers
practice isotope calculations 1 answer key
isotope practice radioactivity1
Abundance Of Isotopes Chem Worksheet 4 3 —
Isotope Practice Worksheet
Isotopes_Worksheet_Answers (1).pdf
Isotopic Abundance Worksheet With Answers
Counting Atoms Practice Worksheet Answers
Here Are Three Isotopes Of An Element:
I Can Use The Atomic Number Of An Element To.
Atomic Mass Of Protons Of Neutrons Of Electrons Isotopie Symbol (Nuclear Form)…
Use Your Periodic Table And The Information Provided.
Related Post: