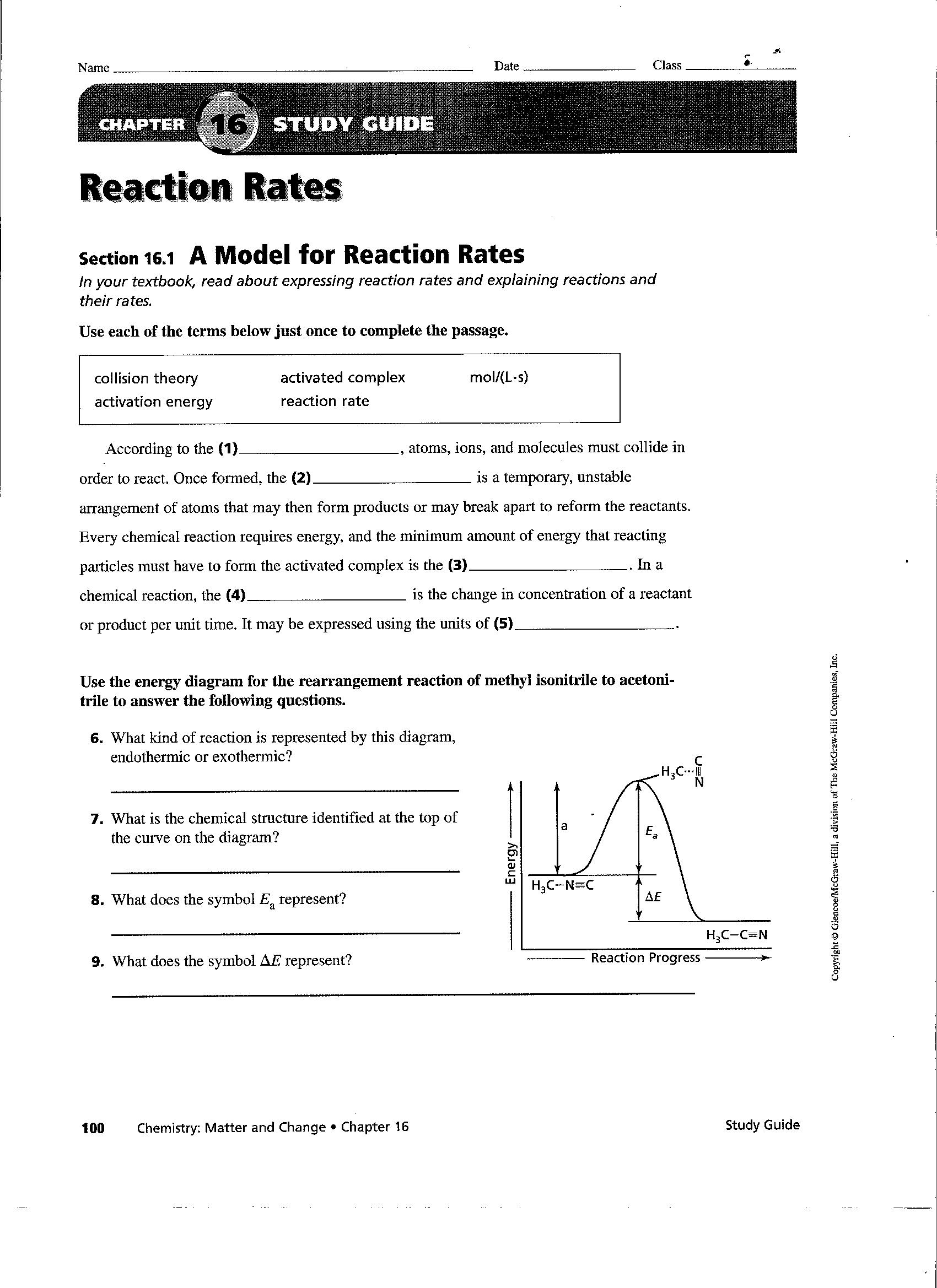
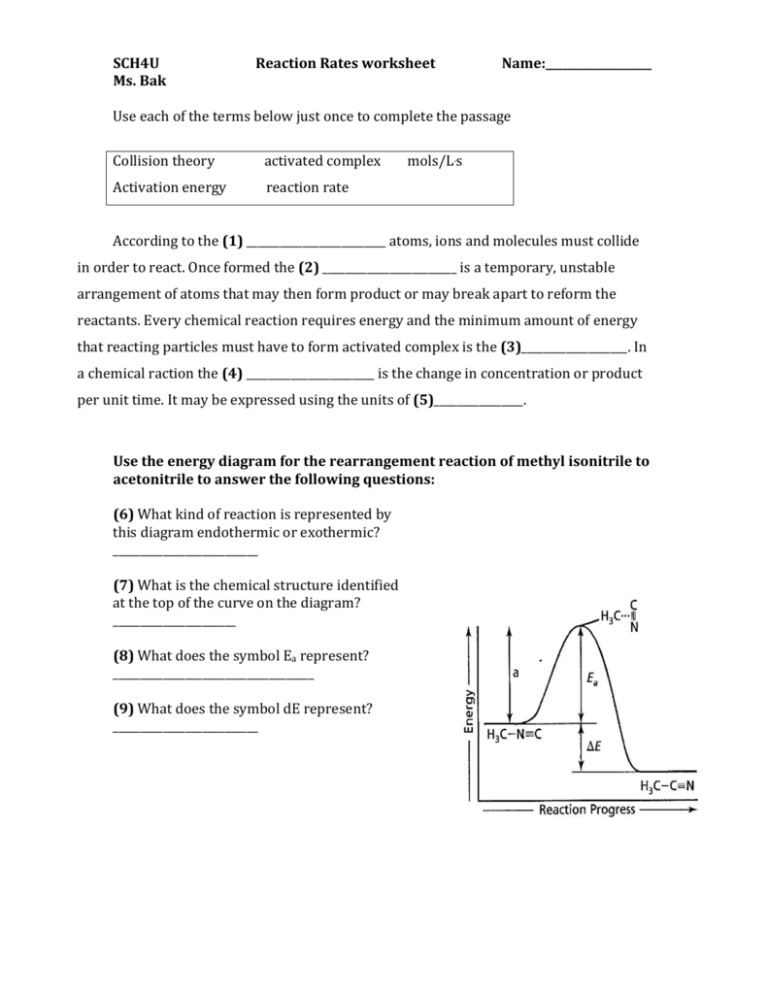
Rates Of Reaction Worksheet Answers
Rates Of Reaction Worksheet Answers - Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the stoichiometric coefficients. Web a study of reaction rate is called chemical kinetic. Answer_____ c) write out the complete ionic equation for the reaction. (1) 2 d (1) 3 d although the option c also shows an increased reaction rate with curve c, it. C6h12o6 & o2 as the reaction proceeds ? Web calculating rates of reaction: For the product being produced ? Web a) calculate the rate of reaction in grams of zn consumed per second. Web 2 worksheets consisting of 30 questions and answers related to calculating average rate of reactions, instantaneous rate of reactions, determining factors affecting rate of reactions (concentration of reactants, temperature, presence of a catalyst, surface area of reactants) and understanding graphs related to the reaction. Finding and using the laws which predict reaction rates. Using the graph below, calculate the rate of the reaction between the second and the fifth minute. Time interval when does the reaction stop? R=0.03 m/s for the product being produced. A sprinkling of hsw stuff as well and a grade ladder. What happens to the concentrations of: Web a study of reaction rate is called chemical kinetic. Plus some application questions involving temperature, concentration, surface area, catalyst. Calculate the average reaction rate for the appearance of oxygen gas during the entire 10 s reaction. Web entify which situation (x or y) would have a higher reaction rate. Another article, rationalising rates, includes tips for teaching rates of. Worksheet on rates of reaction key words, graphs and definitions. R=0.03 m/s for the product being produced. Another article, rationalising rates, includes tips for teaching rates of reaction using graphs. For the product being produced? Reaction rate refers to how quickly or slowly the _______________ disappear and the _______________ appear. Reaction rate refers to how quickly or slowly the _______________ disappear and the _______________ appear. R=0.01 m/s for the product being produced c) the instantaneous rate of reaction at 30 seconds. C) the instantaneous rate of reaction at 30 seconds. The rate law for the reaction involved in inversion of cane sugar is r = k [c 12 h 22. Plus some application questions involving temperature, concentration, surface area, catalyst. Use the equation & the collision theory to explain: Finding and using the laws which predict reaction rates. Choose an answer and hit 'next'. The rate law for the reaction involved in inversion of cane sugar is r = k [c 12 h 22 o 11] [h 2 o]. What is the activation energy for a chemical reaction? A study of reaction _______________ is called chemical ________________. When slope = 0, rate = 0 = reaction is over. The rate law for the reaction involved in inversion of cane sugar is r = k [c 12 h 22 o 11] [h 2 o]. A) increases the speed of a. Reaction rate refers to how quickly or slowly the reactants disappear and the productsappear. When slope = 0, rate = 0 = reaction is over. A chemical reaction is of zero order, when the reaction rate is (where, ca = concentration of reactant) a) ∝ ca b) ∝ 1/ca c) independent of temperature d) none of these answer: Lesson menu. It is measured in terms of the energy of the reactants. Use the equation & the collision theory to explain: Another article, rationalising rates, includes tips for teaching rates of reaction using graphs. For the product being produced ? Web a study of reaction rate is called chemical kinetic. C6h12o6 & o2 as the reaction proceeds ? Web answers to discussion worksheet 6 1. Web rate of chemical reaction. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the stoichiometric coefficients. A study of reaction _______________ is called chemical ________________. Web the factors that affect the rate of chemical reactions are specifically dealt with in this worksheet; For the product being produced ? Calculate the mean rate of reaction from 0 to 30 seconds. For the product being produced? The reaction rate law is reaction rate = k [a] m [b] n where k is a constant. The reaction rate law is reaction rate = k [a] m [b] n where k is a constant. Aa + bb => c. Suitable for additional science c3 or chemistry. Web worksheets are name per work reaction rates, chemistry 12 work 1 1, work reaction rates name, types of reactions work, chapter 16 reaction rates, science term 1 rates of reaction, reaction rates and chemical equilibrium, sample exercise calculating an average rate of reaction. Plus some application questions involving temperature, concentration, surface area, catalyst. H2o + co2 as the reaction proceeds ? The rate law for the reaction involved in inversion of cane sugar is r = k [c 12 h 22 o 11] [h 2 o]. R=0.03 m/s for the product being produced. On the tangent line used. Use the equation & the collision theory to explain: C6h12o6 & o2 as the reaction proceeds ? D) none of these q3: Rates of reactions practice means progress boost your grades with free daily practice questions. Web entify which situation (x or y) would have a higher reaction rate. Web a) calculate the rate of reaction in grams of zn consumed per second. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the stoichiometric coefficients. Calculate the average reaction rate for the appearance of oxygen gas during the entire 10 s reaction. It is measured in terms of the _______________ of the reactants. Some of the worksheets for this concept are rate of reaction work, work reaction rates name, name per work reaction rates, work integrated rate law, chemical kinetics reaction rates, kinetics practice supplemental work key determining, kinetics practice supplemental work. Web product recorded was 24.9 g after 30 seconds. To let us know if it violates our terms and conditions. Rates of reactions practice means progress boost your grades with free daily practice questions. If a reaction is to occur, reacting particles must first collide and this collision must be effective. H2o + co2 as the reaction proceeds ? Situationx situation y situation with a higher reactionrate (xory) f actor affecting the rateof reaction (a) 1 gofsugar(cubes) 1 gofsugar(grains) (b. The rate law for the reaction involved in inversion of cane sugar is r = k [c 12 h 22 o 11] [h 2 o]. Web a) the average rate of reaction over the first 10 seconds. For the product being produced? Calculate the mean rate of reaction from 0 to 30 seconds. For both h 2 o 2 and o 2, compare the average reaction rates during the first 4 seconds of the reaction and the last 4 seconds of the reaction. Then state the factor that affected the rate of reaction in each situation (concentration, surface area, catalyst, temperature). A study of reaction _______________ is called chemical ________________. Calculate the average reaction rate for the appearance of oxygen gas during the entire 10 s reaction. Web calculating rates of reaction: Aa + bb => c. Web worksheets are name per work reaction rates, chemistry 12 work 1 1, work reaction rates name, types of reactions work, chapter 16 reaction rates, science term 1 rates of reaction, reaction rates and chemical equilibrium, sample exercise calculating an average rate of reaction.Reaction Rates Worksheet
13 Worksheet Reaction Rates Answer /
13 Worksheet Reaction Rates Answer /
Rates Of Chemical Reactions Worksheet Escolagersonalvesgui
reaction rate worksheet key
Answers Unit 1 Rates of Reactions CfE Higher Chemistry Revision
Reaction Rates worksheet
Rate of Reaction f5(Worksheet) Reaction Rate Chemical Reactions
Rates Of Reaction Worksheet Sky Hut
13 Best Images of Worksheet Reaction Rates Answer Worksheet Measuring
What Happens To The Concentrations Of:
Finding And Using The Laws Which Predict Reaction Rates.
Web During The Entire 10 S Reaction.
The Reaction Rate Law Is Reaction Rate = K [A] M [B] N Where K Is A Constant.
Related Post: