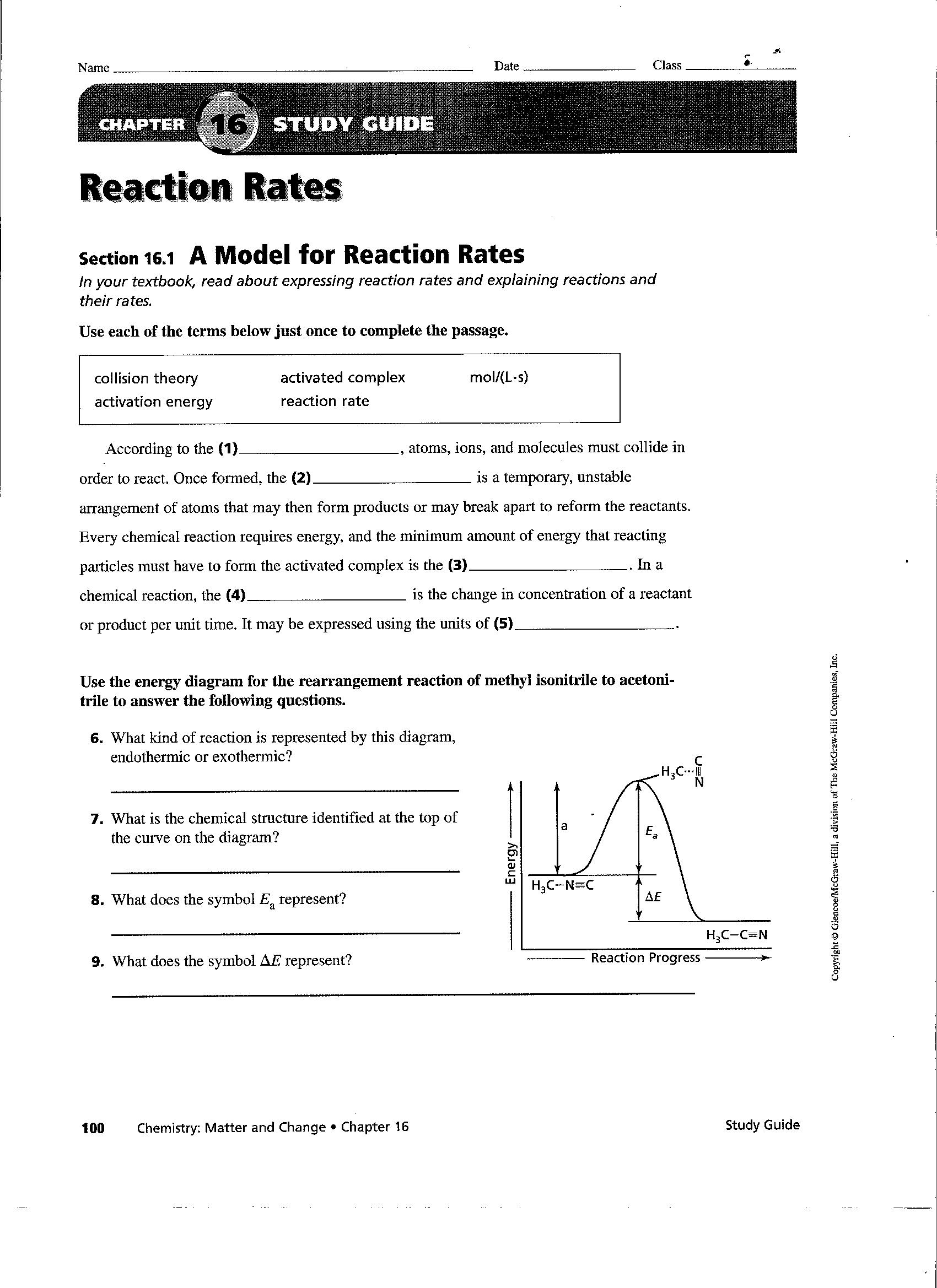
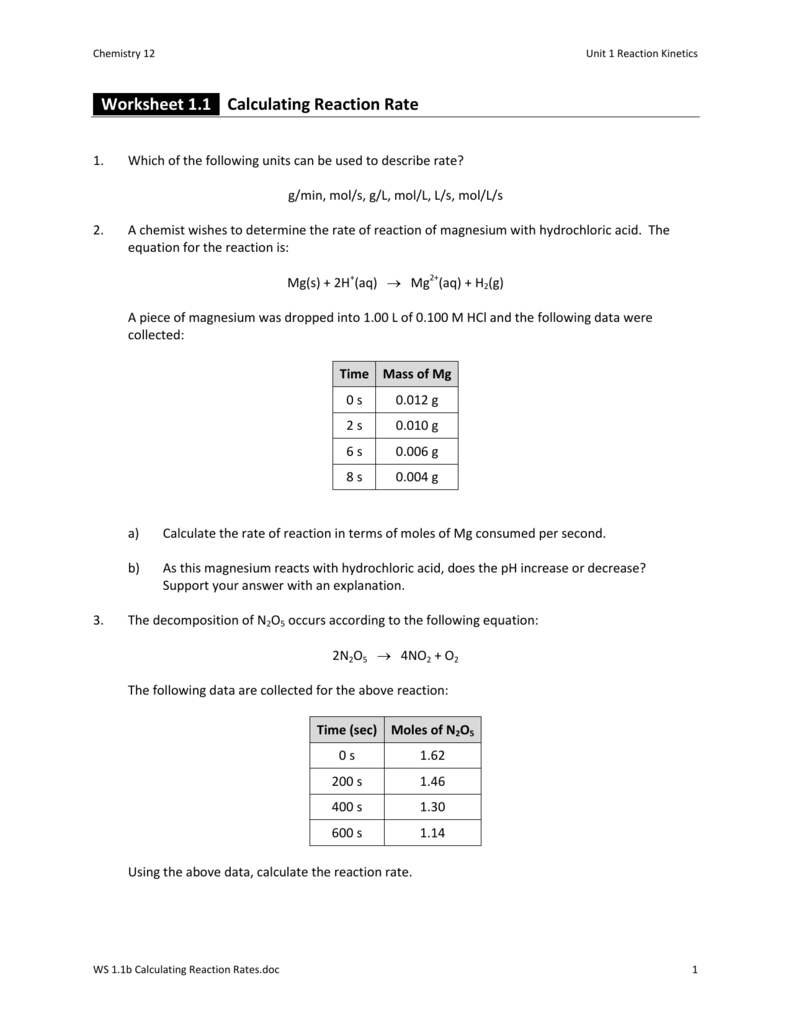
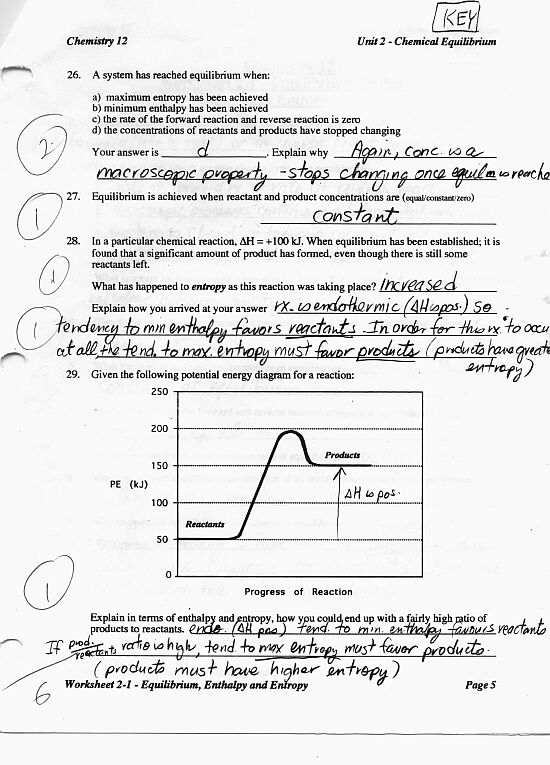
Reaction Rates And Equilibrium Worksheet
Reaction Rates And Equilibrium Worksheet - Web reaction involves solids or pure liquids, they are not included in the equilibrium expression. Key topics you'll be covering include a. At a particular time, the following concentrations are measured: Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the. Web rates of reaction, rate equations and k c (k p would be helpful but it is explained in the worksheet). [co] = 0.034m [co2] = 3.6 ×. Ke ke= (co₂)² [co] (or b. Web reaction rates and equilibrium reaction rates the rate of a chemical reaction is the change in the amount of a reactant or product of the reaction with time. Calculate the equilibrium constant for the reaction equation at 527°c. Web this escape room has students work through rooms to learn about reaction rates and equilibrium. To be able to write down an expression for a reactions rate and relate that to the rate law for that reaction. The rate of the forward reaction is equal to the rate of the reverse reaction. Write the equilibrium expression for the following reactions: Web rates of reaction & equilibrium unit bundle contains 25 completed student pages, 60 task. Design experiments with different reactions, concentrations, and. Topics covered include kinetic theory, activation energy, reaction. The rate of the forward reaction is equal to the rate of the reverse reaction. Some of the worksheets for this concept are potential energy diagram work answers, chemistry. Rationale this activity demonstrates the links between the topics of rates of. Reaction rates and rate laws objectives: Web q8.5 keq is 7.7 × 10 − 15 for the reaction 2co(g) ⇌ c(s) + co2(g). Write the equilibrium expression for the following reactions: Web this quiz/worksheet combination will assess how much you know about determining rate equation, rate law constant and reaction order. Web this activity demonstrates the links between the topics. Web at equilibrium [pcl5]= 0,10 mol.dm−3, [pcl3]= 0,15 mol.dm−3 and [cl2]= 0,37 mol.dm−3. To be able to write down an expression for a reactions rate and relate that to the rate law for that reaction. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the. Rationale this activity demonstrates. Web this quiz/worksheet combination will assess how much you know about determining rate equation, rate law constant and reaction order. Topics covered include kinetic theory, activation energy, reaction. Web reaction rates and equilibrium reaction rates the rate of a chemical reaction is the change in the amount of a reactant or product of the reaction with time. Read each of. To be able to write down an expression for a reactions rate and relate that to the rate law for that reaction. Web heated at 527°c in a reaction vessel. Reaction rate & equilibrium worksheet 1. Using the graph below, calculate the rate of the reaction between the second and the fifth minute. Calculate the equilibrium constant for the reaction. Explore what makes a reaction happen by colliding atoms and molecules. Web this activity demonstrates the links between the topics of rates of reaction and the equilibrium law. Web what affects the rate of a reaction? This unit covers what reaction. Rationale this activity demonstrates the links between the topics of rates of. It provides students with an explanation of the equilibrium law and. Ke ke= (co₂)² [co] (or b. Rationale this activity demonstrates the links between the topics of rates of. Key topics you'll be covering include a. The fundamental reason for this is that the concentration of a solid or pure liquid cannot. At a particular time, the following concentrations are measured: Reaction rates and rate laws objectives: Tick one of the boxes to show that you think the statement is true, false or that you are unsure whether it. To be able to write down an expression for a reactions rate and relate that to the rate law for that reaction. Write. [co] = 0.034m [co2] = 3.6 ×. At equilibrium, the concentration of co(g) was found to be 0.046 m. This unit covers what reaction. To be able to write down an expression for a reactions rate and relate that to the rate law for that reaction. Web this activity demonstrates the links between the topics of rates of reaction and. Web q8.5 keq is 7.7 × 10 − 15 for the reaction 2co(g) ⇌ c(s) + co2(g). Web rates of reaction, rate equations and k c (k p would be helpful but it is explained in the worksheet). Web what affects the rate of a reaction? Web a series of free high school chemistry video lessons. Topics covered include kinetic theory, activation energy, reaction. Reaction rate & equilibrium worksheet 1. This unit covers what reaction. Explore what makes a reaction happen by colliding atoms and molecules. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the. The fundamental reason for this is that the concentration of a solid or pure liquid cannot. Write the equilibrium expression for the following reactions: Web reaction rates and equilibrium reaction rates the rate of a chemical reaction is the change in the amount of a reactant or product of the reaction with time. Rationale this activity demonstrates the links between the topics of rates of. Calculate the equilibrium constant for the reaction equation at 527°c. Tick one of the boxes to show that you think the statement is true, false or that you are unsure whether it. Web reaction rates and equilibrium worksheet curated and reviewed by lesson planet in this equilibrium instructional activity, students solve 8 problems to find the equilibrium. At equilibrium, the concentration of co(g) was found to be 0.046 m. [co] = 0.034m [co2] = 3.6 ×. No further changes occur in the concentrations of reactants and. Web reaction involves solids or pure liquids, they are not included in the equilibrium expression. To be able to write down an expression for a reactions rate and relate that to the rate law for that reaction. Web reaction involves solids or pure liquids, they are not included in the equilibrium expression. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the. Key topics you'll be covering include a. This unit covers what reaction. Web what affects the rate of a reaction? Web this activity demonstrates the links between the topics of rates of reaction and the equilibrium law. The rate of the forward reaction is equal to the rate of the reverse reaction. Write the equilibrium expression for the following reactions: Web heated at 527°c in a reaction vessel. Web this escape room has students work through rooms to learn about reaction rates and equilibrium. Web at equilibrium [pcl5]= 0,10 mol.dm−3, [pcl3]= 0,15 mol.dm−3 and [cl2]= 0,37 mol.dm−3. Web reaction rates and equilibrium reaction rates the rate of a chemical reaction is the change in the amount of a reactant or product of the reaction with time. [co] = 0.034m [co2] = 3.6 ×. Web this quiz/worksheet combination will assess how much you know about determining rate equation, rate law constant and reaction order. Web a series of free high school chemistry video lessons.Worksheet Chemical Reaction Rates & Equilibrium
Chapter 18 Reaction Rates And Equilibrium Worksheet Answers worksheet
13 Worksheet Reaction Rates Answer /
reaction rates and equilibrium worksheet
Rates Of Reaction Worksheet Ivuyteq
Reaction Rates And Equilibrium Worksheets
Writing An Equilibrium Expression Chem Worksheet 18 2 Answers
chemical equilibrium worksheet 1
Equilibrium Expressions Worksheet worksheet
Dynamic Equilibrium
Using The Graph Below, Calculate The Rate Of The Reaction Between The Second And The Fifth Minute.
The Fundamental Reason For This Is That The Concentration Of A Solid Or Pure Liquid Cannot.
At Equilibrium, The Concentration Of Co(G) Was Found To Be 0.046 M.
Reaction Rates And Rate Laws Objectives:
Related Post: