Redox Reactions Worksheet
Redox Reactions Worksheet - Web a redox reaction is a chemical reaction that transfers electrons between two reactants. 1) mg + 2hcl ( mgcl2 + h2 2) 2fe + 3v2o3 ( fe2o3 + 6vo 3) 2kmno4 + 5kno2 + 3h2so4 ( 2mnso4 + 3h2o + 5kno3 + k2so4 4) k2cr2o7 + 3sncl2 + 14hcl ( 2crcl3 + 3sncl4 + 2kcl + 7h2o Use the rules for balancing redox reactions to balance the equation. Second & third laws of thermodynamics. Determine what is oxidized and what agent and the reducing agent, also. Web balance each half reaction in basic solution. Oxidation is the loss of electrons and the gaining of a positive charge. Next, identify the oxidation states of the elements involved in the reaction. Web balance the following redox reactions: Web balancing redox reactions oxidation/reduction (redox) reactions can be balanced using the oxidation state changes, as seen in the previous example. Multiply by some integer to make electrons (lost) = electrons (gained) step 6: 1) mg + 2hcl ( mgcl2 + h2 2) 2fe + 3v2o3 ( fe2o3 + 6vo 3) 2kmno4 + 5kno2 + 3h2so4 ( 2mnso4 + 3h2o + 5kno3 + k2so4 4) k2cr2o7 + 3sncl2 + 14hcl ( 2crcl3 + 3sncl4 + 2kcl + 7h2o Ascorbic acid (c. Worksheets are chapter 20 work redox, work, work 1 redox reactions, 2 5 redox reactions practice work with answers, balancing redox reactions learn and practice, oxidation reduction handout, , oxidation and reduction workbook revised 1a. Reduction is the gain of electrons and the gain of negative charge. Balance the atom undergoing redox changes, if necessary. Add the number of electrons. Balance h by adding h + step 5: Web redox reaction worksheet subject: Oxidation is the loss of electrons and the gaining of a positive charge. Web redox worksheets and online activities. 2015 ap chemistry free response 1d. This electron transfer can be identified by the change in the oxidation states of the reacting species. Balance the charge or oxidation number with electrons step 3: Add half equations and cancel substances on both sides First, you need to gather all the necessary information to complete the form, such as the type of redox reaction, the reactants, and products.. Web balancing redox reactions oxidation/reduction (redox) reactions can be balanced using the oxidation state changes, as seen in the previous example. Identify the oxidizing 2sr + o2 2sro 2li + s li2s 2cs + br2 2csbr 3mg + n2 mg3n2 4fe + 3o2 2fe2o3 cl2 + 2nabr 2nacl + br2 si + 2f2 sif4 2ca + o2 2cao mg +. Balance the charge or oxidation number with electrons step 3: Web balance each half reaction in basic solution. Web a redox reaction is a chemical reaction that transfers electrons between two reactants. 2015 ap chemistry free response 1d. Determine what is oxidized and what agent and the reducing agent, also. Determine what is oxidized and what agent and the reducing agent, also. Other file previews docx, 15.41 kb a quick sheet to assess student understanding of the processes. Web a series of free high school chemistry video lessons. Can they identify the various key points given an equation. First, you need to gather all the necessary information to complete the. Balance h by adding h + step 5: Next, identify the oxidation states of the elements involved in the reaction. Web balance each half reaction in basic solution. 2015 ap chemistry free response 1d. Web it consists of the following steps: Web balancing redox reactions worksheet 1 balance each redox reaction in acid solution. Add half equations and cancel substances on both sides Determine what is oxidized and what agent and the reducing agent, also. Redox reaction from dissolving zinc in copper sulfate. Balance the charge or oxidation number with electrons step 3: 1) mg + 2hcl ( mgcl2 + h2 2) 2fe + 3v2o3 ( fe2o3 + 6vo 3) 2kmno4 + 5kno2 + 3h2so4 ( 2mnso4 + 3h2o + 5kno3 + k2so4 4) k2cr2o7 + 3sncl2 + 14hcl ( 2crcl3 + 3sncl4 + 2kcl + 7h2o Also assesses their ability to balance equations creative commons sharealike This is best shown by working. Shorthand notation for galvanic/voltaic cells. We can “see” these changes if we assign oxidation numbers to the reactants and products. This electron transfer can be identified by the change in the oxidation states of the reacting species. Web balance each half reaction in basic solution. Web how to fill out redox practice worksheet answers. Next, identify the oxidation states of the elements involved in the reaction. Add half equations and cancel substances on both sides Web balance the following redox reactions: Reduction is the gain of electrons and the gain of negative charge. Web a series of free high school chemistry video lessons. Also assesses their ability to balance equations creative commons sharealike Oxidation and reduction occur simultaneously in order to conserve charge. Add the number of electrons that correspond to the change in oxidation state. Fuel combustion, metal corrosion, and even photosynthesis and cellular respiration all involve oxidation and reduction. Other file previews docx, 15.41 kb a quick sheet to assess student understanding of the processes. Electrodes and voltage of galvanic cell. Ascorbic acid (c \text {}_ {6} 6 h \text {}_ {8} 8 o \text {}_ {6} 6) is a common antioxidant that protects our bodies against radicals. Use the rules for balancing redox reactions to balance the equation. Multiply by some integer to make electrons (lost) = electrons (gained) step 6: Web redox reaction worksheet subject: Multiply by some integer to make electrons (lost) = electrons (gained) step 6: Use the rules for balancing redox reactions to balance the equation. Can they identify the various key points given an equation. Web balancing redox reactions worksheet 1 balance each redox reaction in acid solution. Determine what is oxidized and what agent and the reducing agent, also. Web balance each half reaction in basic solution. Fuel combustion, metal corrosion, and even photosynthesis and cellular respiration all involve oxidation and reduction. Web balancing redox reactions oxidation/reduction (redox) reactions can be balanced using the oxidation state changes, as seen in the previous example. Electrodes and voltage of galvanic cell. Web redox reaction worksheet subject: Also assesses their ability to balance equations creative commons sharealike Add the number of electrons that correspond to the change in oxidation state. 1) mg + 2hcl ( mgcl2 + h2 2) 2fe + 3v2o3 ( fe2o3 + 6vo 3) 2kmno4 + 5kno2 + 3h2so4 ( 2mnso4 + 3h2o + 5kno3 + k2so4 4) k2cr2o7 + 3sncl2 + 14hcl ( 2crcl3 + 3sncl4 + 2kcl + 7h2o Shorthand notation for galvanic/voltaic cells. This is best shown by working an example. Redox reaction from dissolving zinc in copper sulfate.redox reactions worksheet answers
Redox Reactions Worksheet Answer Key Kidsworksheetfun
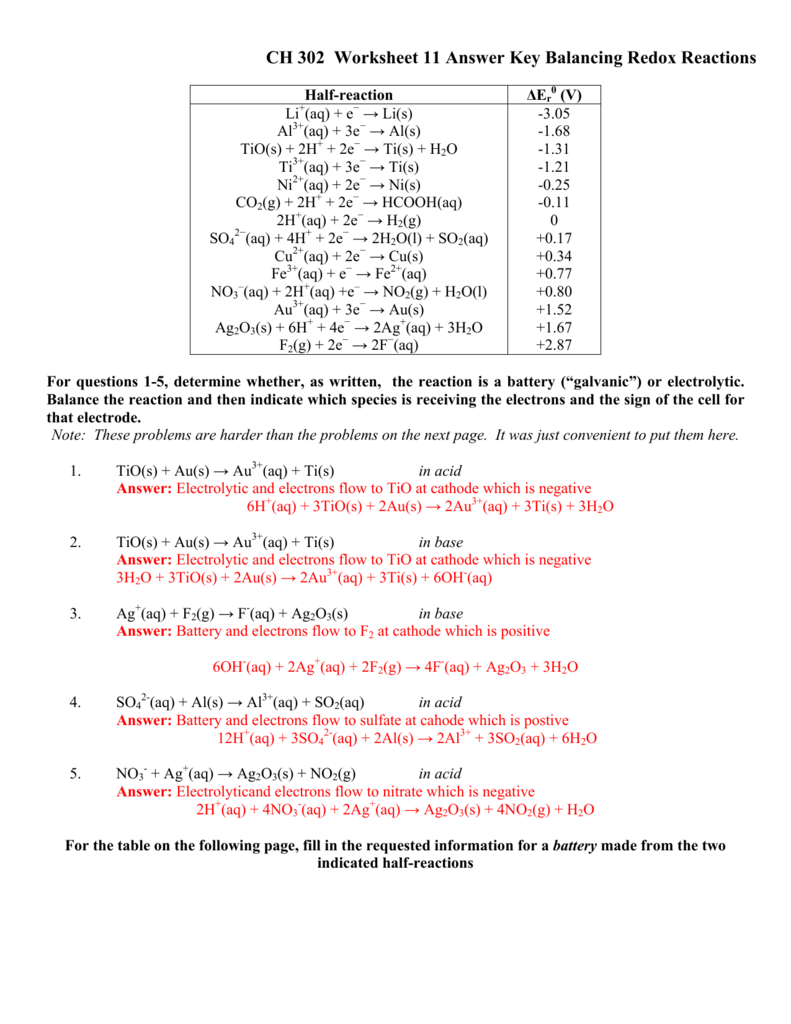
CH 302 Worksheet 11 Answer Key Balancing Redox Reactions
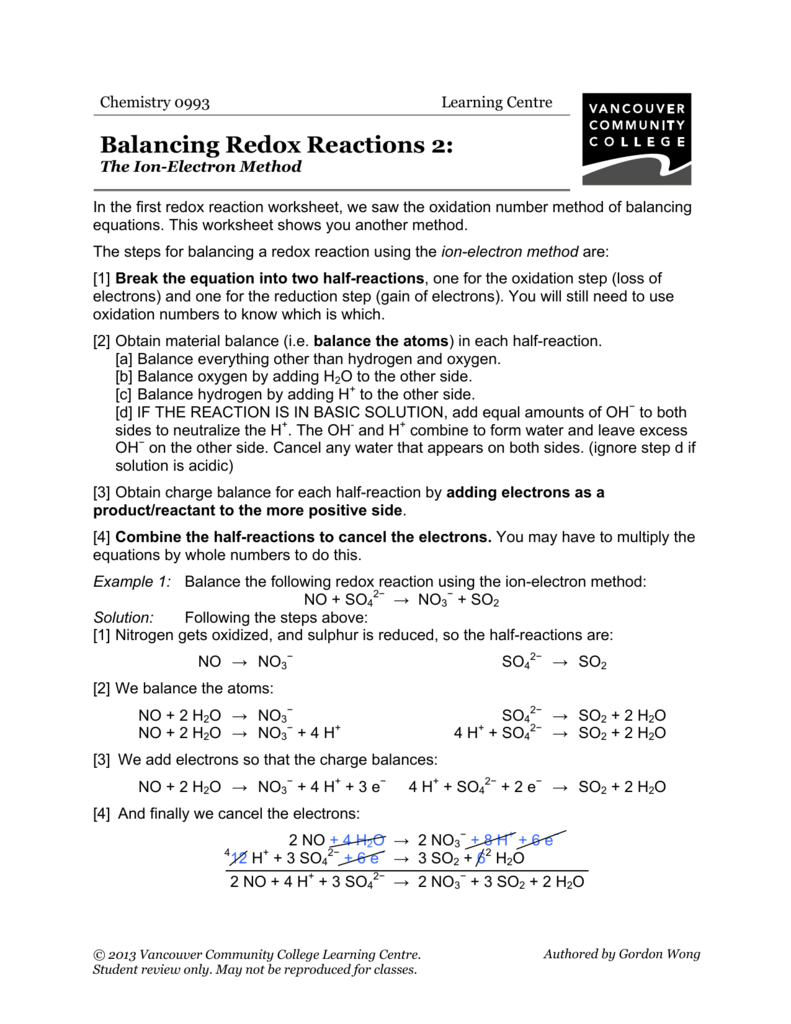
Balancing Redox Reactions 2 VCC Library
Oxidation Reduction Worksheet Answers
Balancing Redox Reactions Worksheet properinspire
Oxidation And Reduction Worksheet Redox reactions, Chemistry
redox reactions worksheets
Redox Reactions Worksheet With Answers Printable Worksheets and
Balancing Redox Reactions Worksheet With Answers
Oxidation Is The Loss Of Electrons And The Gaining Of A Positive Charge.
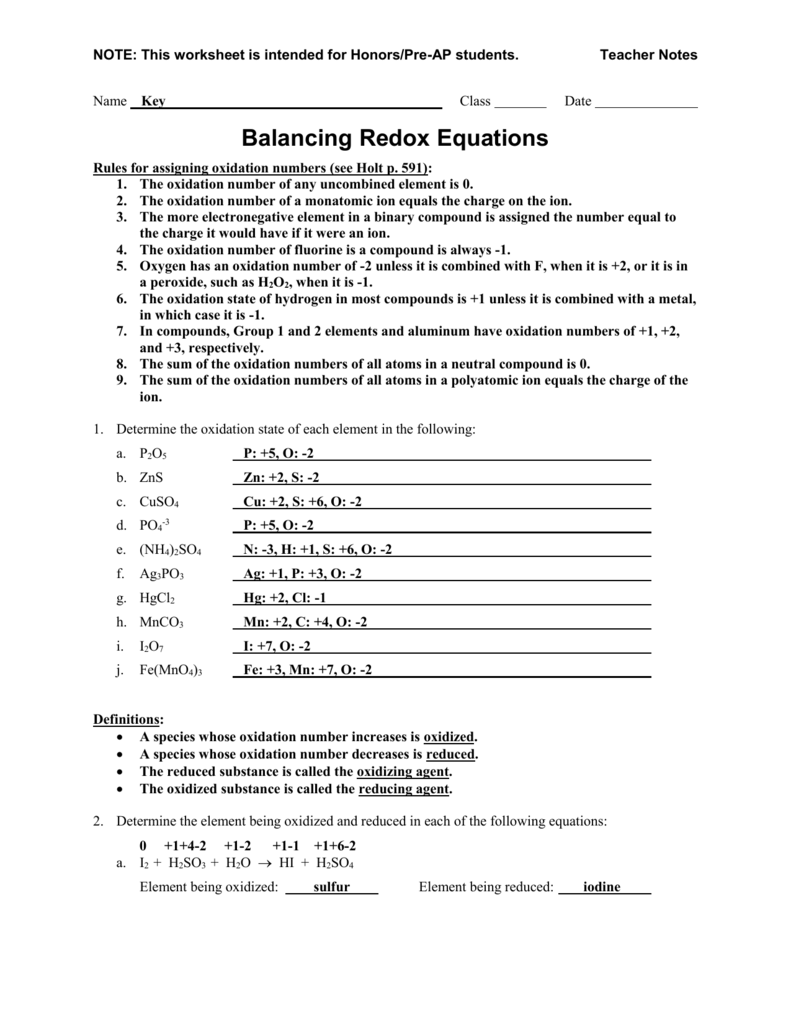
Identify The Oxidizing 2Sr + O2 2Sro 2Li + S Li2S 2Cs + Br2 2Csbr 3Mg + N2 Mg3N2 4Fe + 3O2 2Fe2O3 Cl2 + 2Nabr 2Nacl + Br2 Si + 2F2 Sif4 2Ca + O2 2Cao Mg + 2Hcl Mgcl2 + H2
Reduction Is The Gain Of Electrons And The Gain Of Negative Charge.
We Can “See” These Changes If We Assign Oxidation Numbers To The Reactants And Products.
Related Post: