Solubility And Concentration Worksheet Answers
Solubility And Concentration Worksheet Answers - Table salt, baking soda, table sugar., identify how solutions can be classified based on solubility and more. Baso4 with ksp = 1.5 × 10 ‐ 9. The topics covered in these assessments are factors that affect solubility, rate of dissolving, acids, bases, titrations, total ionic equation, net ionic equation, solving for various concentrations and solution terminology. Agcn with ksp = 2.0 × 10 ‐ 12. Fes with ksp = 3.7 × 10 ‐ 19. Web to measure concentration, you compare the amount of solute to the total amount of solution. 10 moles of potassium hydroxide in 5.16 l of solution. How many milliliters of 0.20 m alcl3 solution would be necessary to precipitate all of the ag+ from 45ml of a 0.20 m agno3 solution? Watch videos and use nagwa’s tools and apps to help students achieve their full potential. Why is solubility useful in identifying substances? Ach of the following so. Web in this unit test, students will answer questions and work problems about: Watch videos and use nagwa’s tools and apps to help students achieve their full potential. As always, show your work! What volume of concentrated ammonium hydroxide is needed. Circle the letters that identify how solutions can be classified based on solubility. Be able to write balanced ionic and net ionic equations for reactions occurring when electrolyte solutions are mixed. Ach of the following solutions: Be able to predict reactions of mixed electrolyte solutions. Web study with quizlet and memorize flashcards containing terms like solubility, saturated solution, unsaturated solution. [25.4%) 2) calculate the molarity of a solution that contains 18.9 grams of sodium hydroxide in 3.67 l of solution. If you want some element x whose concentration is equal to 0.20 m to exist entirely in this solution, what will be the highest ph possible for this mixture? Web calculate the solubility in moles/l of each of three salts. Solution that has only a little solute dissolved in a certain amount of solvent. The topics covered in these assessments are factors that affect solubility, rate of dissolving, acids, bases, titrations, total ionic equation, net ionic equation, solving for various concentrations and solution terminology. Circle the letters that identify how solutions can be classified based on solubility. Download just the. Calculate the concentration in e. Circle the letters that identify how solutions can be classified based on solubility. Web study with quizlet and memorize flashcards containing terms like solubility, saturated solutions, unsaturated solutions and more. Web read this passage from the text and answer the questions that follow. Web it includes 20 solutions and solubility tests and is 71 pages. Which of the following salts has the greatest molar solubility in pure water? Watch videos and use nagwa’s tools and apps to help students achieve their full potential. What molarity of diluted sodium hydroxide would be obtained. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric acid. List the following solutes in order from most soluble to. (b) 100.0 ml of 3.8 × 10 −5 m nacn, the minimum lethal concentration of sodium cyanide in blood serum. Web use the table below to help you answer the following questions. Web find and create gamified quizzes, lessons, presentations, and flashcards for students, employees, and everyone else. Web showing 8 worksheets for solubility and concentration. Web read this passage. Predict how solution concentration will change for any action (or combination of actions) that adds or removes water, solute, or solution, and explain why. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric acid. Web learn about solutions, concentration, and solubility through this lesson. The amount of solute dissolved in the solvent. Which of the following salts. Web study with quizlet and memorize flashcards containing terms like define solubility, list the following solutes in order from most soluble to least soluble in water; What volume of concentrated hydrochloric acid is needed. Which of the following salts has the greatest molar solubility in pure water? The topics covered in these assessments are factors that affect solubility, rate of. Watch videos and use nagwa’s tools and apps to help students achieve their full potential. Web question answer what is solubility? Download just the worksheet or there’s a copy included in the packet. (b) 100.0 ml of 3.8 × 10 −5 m nacn, the minimum lethal concentration of sodium cyanide in blood serum. Why is solubility useful in identifying substances? Circle the letters that identify how solutions can be classified based on solubility. You can identify a substance by its solubility because it is a characteristic property of matter. Ph = 4.00 buffer also, calculate these solubilities in g solid/l solution. Web terms in this set (8) concentration. To make 250 ml of. What volume of concentrated hydrochloric acid is needed. 0.5 moles of calcium chloride in 485 ml of solution. [25.4%) 2) calculate the molarity of a solution that contains 18.9 grams of sodium hydroxide in 3.67 l of solution. Web study with quizlet and memorize flashcards containing terms like solubility, saturated solution, unsaturated solution and more. Which of the following salts has the greatest molar solubility in pure water? Web find and create gamified quizzes, lessons, presentations, and flashcards for students, employees, and everyone else. Worksheets are section 2 solubility and concentration, solubility product work, solubility work. Find the number of moles of solute in e. What volume of concentrated ammonium hydroxide is needed. Solution that has a lot of solute dissolved in the solvent. Web question answer what is solubility? (you make the amount of solute be less in your solvent) concentrated solution. To make 3.0 l of 1.0 m hcl? How many milliliters of 0.20 m alcl3 solution would be necessary to precipitate all of the ag+ from 45ml of a 0.20 m agno3 solution? Alcl3(aq) + 3agno3(aq) al(no3)3(aq) + 3agcl(s). Why is solubility useful in identifying substances? Fes with ksp = 3.7 × 10 ‐ 19. (a) 2.00 l of 18.5 m h 2 so 4, concentrated sulfuric acid. Web study with quizlet and memorize flashcards containing terms like solubility, saturated solutions, unsaturated solutions and more. Calculate the concentration in e. Web study with quizlet and memorize flashcards containing terms like define solubility, list the following solutes in order from most soluble to least soluble in water; What volume of concentrated ammonium hydroxide is needed. Solution that has only a little solute dissolved in a certain amount of solvent. Web showing 8 worksheets for solubility and concentration. Predict how solution concentration will change for any action (or combination of actions) that adds or removes water, solute, or solution, and explain why. Web learn about solutions, concentration, and solubility through this lesson. (b) 100.0 ml of 3.8 × 10 −5 m nacn, the minimum lethal concentration of sodium cyanide in blood serum. Design a procedure for creating a solution of a given concentration. Web be able to predict the solubility or insolubility of simple ionic compounds in water. Web solubility, ksp worksheet 1. Worksheets are section 2 solubility and concentration, solubility product work, solubility work.Article Solubility And Molarity Worksheet Answers
Solubility Curve Worksheet
Solubility, Ksp Worksheet 4
solubility rules worksheet answers
Substances Mixtures And Solubility Worksheet Answers Escolagersonalvesgui
20++ Solubility Worksheet Answer Key
10 Best Images of Chemistry Answer Worksheet 3.1 Solubility Curves
14 Best Images of Chemistry Solubility Worksheet Theory of Evolution
Solubility And Concentration Worksheet Answers
20++ Solubility Worksheet Answer Key
Mg(Oh)2 With Ksp = 9.0 × 10 ‐ 12.
Circle The Letters That Identify How Solutions Can Be Classified Based On Solubility.
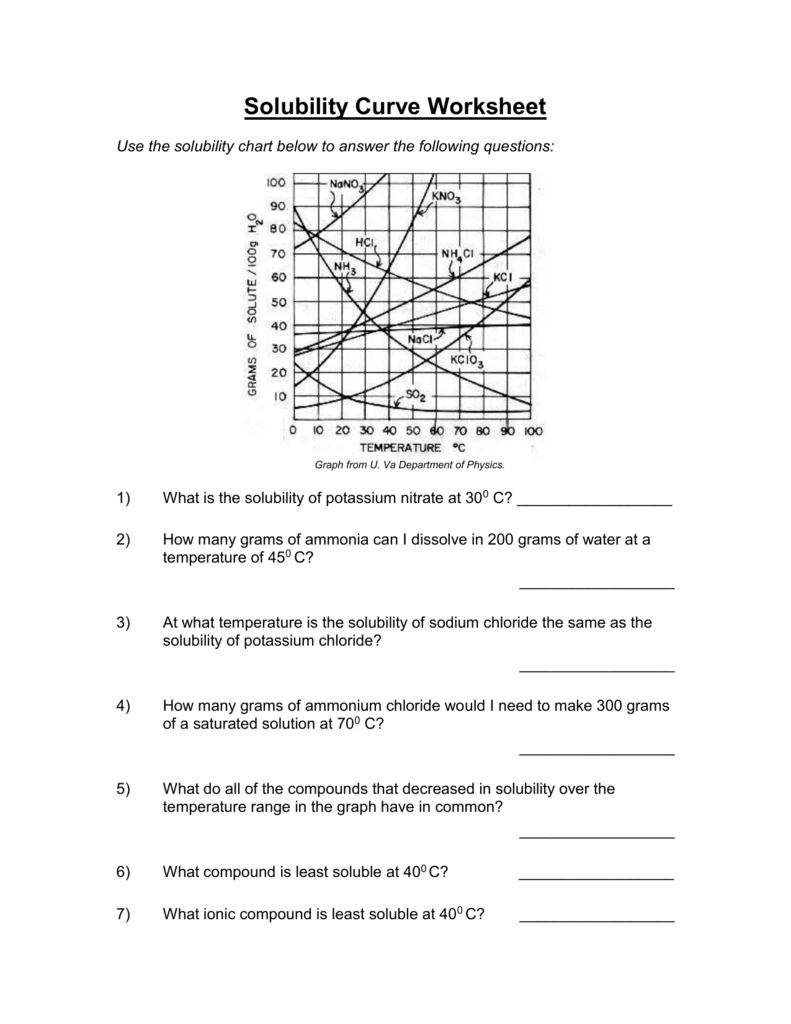
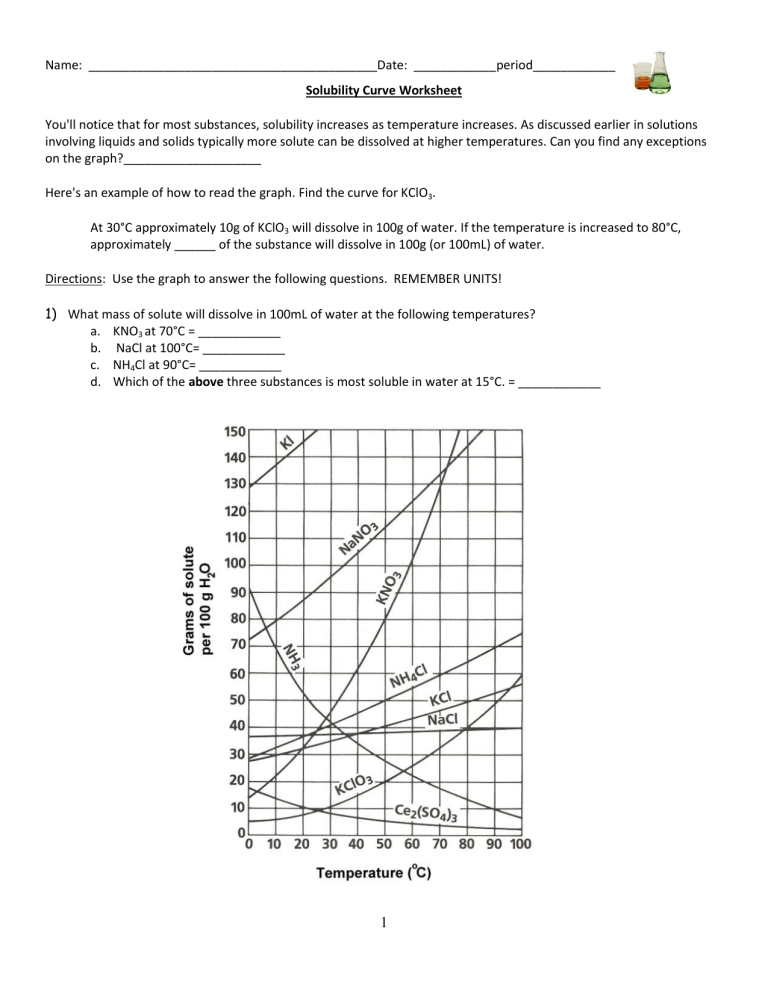
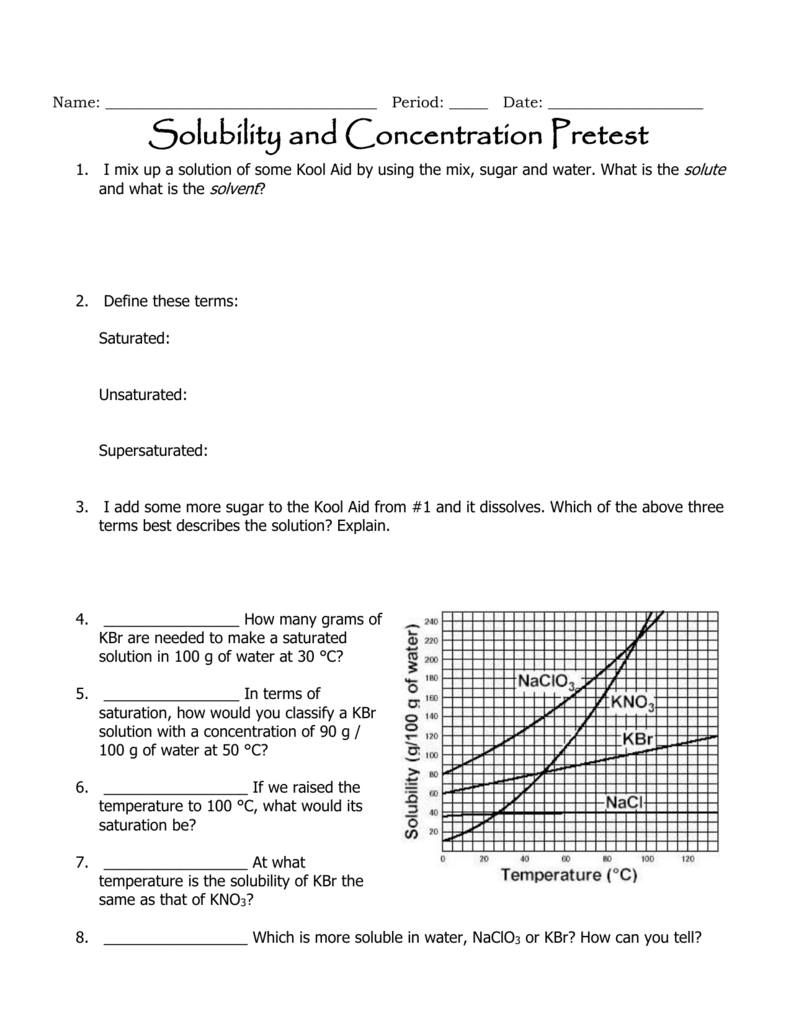
(1) Solutions And The Factors That Affect Solubility, (2) Use A Solubility Curve To Determine Solubility At Various Temperatures, (3) Use A Solubility Curve To Determine If Solutions Are Saturated, Unsaturated, Or Supersaturated At Different Temperatures, (4) Determine.
Web Find And Create Gamified Quizzes, Lessons, Presentations, And Flashcards For Students, Employees, And Everyone Else.
Related Post: