Specific Heat And Calorimetry Worksheet
Specific Heat And Calorimetry Worksheet - Web heat capacity and calorimetry. A piece of lead (c = 130 j/kg/oc) at 82.0 oc is. Web calorimetry problems worksheet 1 note: Web heat capacity, molar heat capacity, and specific heat the heat capacity, c, is the amount of heat, q, required to raise the temperature, δ t, of an object by 1 o c. Calculate and interpret heat and related properties using typical calorimetry data. Since specific heat can be. Web which kind of substance needs more energy to undergo an increase of 5 oc, something with a high or low specific heat? Web do you need a worksheet activity to help gather information about student understanding of specific heat and calorimetry? One technique we can use to measure the amount of. Name:______________________________ pd_____specific heat and calorimetry. Name:______________________________ pd_____specific heat and calorimetry. Web do you need a worksheet activity to help gather information about student understanding of specific heat and calorimetry? Web heat capacity, molar heat capacity, and specific heat the heat capacity, c, is the amount of heat, q, required to raise the temperature, δ t, of an object by 1 o c. Specific heat capacityreal. Calculate and interpret heat and related properties using typical calorimetry data. The specific heat capacity of water is 4.184 j/g•°c, and the specific heat capacity of mercury is 0.139 j/g•°c. Web specific heat and calorimetry worksheet key.pdf. Calculate the specific heat capacity of the metal. Web you may be offline or with limited connectivity. Calculate the specific heat capacity of the metal. Web you may be offline or with limited connectivity. How much heat is required to raise the temperature of 67.0g of water from 25.7(c to 66.0(c? Web this worksheet set guides students through the following topics: Web specific heat and calorimetry worksheet key.pdf. The initial temperature of the water is 23.00°c. G iron rod is heated to a temperature t_1 t 1 and then dropped into 20.\; Web calorimetry problems worksheet 1 note: The specific heat capacity of water is 4.184 j/g•°c, and the specific heat capacity of mercury is 0.139 j/g•°c. Web which kind of substance needs more energy to undergo an. Calculate and interpret heat and related properties using typical calorimetry data. Web explain the technique of calorimetry. Web do you need a worksheet activity to help gather information about student understanding of specific heat and calorimetry? Web heat capacity, molar heat capacity, and specific heat the heat capacity, c, is the amount of heat, q, required to raise the temperature,. Displaying specific heat and calorimetry worksheet key.pdf. Specific heat capacityreal examples of specific heatcalorimetry (including parts of a. Web do you need a worksheet activity to help gather information about student understanding of specific heat and calorimetry? Calculate the specific heat capacity of the metal. Web this worksheet set guides students through the following topics: G iron rod is heated to a temperature t_1 t 1 and then dropped into 20.\; In this chemistry lab, students will use a simple calorimeter and laboratory balance to determine the. Web specific heat of a metal chemistry lab. Web calorimetry problems worksheet 1 note: The specific heat of water = 4.18 j/goc a reaction takes place in a. Calculate and interpret heat and related properties using typical calorimetry data. The initial temperature of the water is 23.00°c. Web what is the specific heat of the mystery liquid? Web do you need a worksheet activity to help gather information about student understanding of specific heat and calorimetry? Name:______________________________ pd_____specific heat and calorimetry. Web do you need a worksheet activity to help gather information about student understanding of specific heat and calorimetry? Web heat capacity and calorimetry. Web specific heat and calorimetry worksheet a calorimeter has a heat capacity of 4.18 kj/g oc. The specific heat capacity of water is 4.184 j/g•°c, and the specific heat capacity of mercury is 0.139 j/g•°c. Web. Web heat capacity, molar heat capacity, and specific heat the heat capacity, c, is the amount of heat, q, required to raise the temperature, δ t, of an object by 1 o c. Web specific heat and calorimetry worksheet a calorimeter has a heat capacity of 4.18 kj/g oc. Web specific heat of a metal chemistry lab. Web the specific. The specific heat of water = 4.18 j/goc a reaction takes place in a calorimeter containing 400.0 g of water at an initial temperature. Calculate the specific heat capacity of the metal. Web do you need a worksheet activity to help gather information about student understanding of specific heat and calorimetry? G iron rod is heated to a temperature t_1 t 1 and then dropped into 20.\; In this chemistry lab, students will use a simple calorimeter and laboratory balance to determine the. Web the specific heats of gases depend on what is maintained constant during the heating—typically either the volume or the pressure. Web heat capacity and calorimetry. Web you may be offline or with limited connectivity. Specific heat capacityreal examples of specific heatcalorimetry (including parts of a. Web what is the specific heat of the mystery liquid? Web specific heat and calorimetry (heat lost=heat gained) worksheet 1. Web specific heat and calorimetry worksheet key.pdf. Web heat capacity, molar heat capacity, and specific heat the heat capacity, c, is the amount of heat, q, required to raise the temperature, δ t, of an object by 1 o c. Complete combustion of 1.00 g of hydrogen in this calorimeter causes. In this specific heat and calorimetry instructional activity, students are given specific. Displaying specific heat and calorimetry worksheet key.pdf. Web explain the technique of calorimetry. Name:______________________________ pd_____specific heat and calorimetry. G of water at a lower temperature t_2 t 2 in. How much heat is required to raise the temperature of 67.0g of water from 25.7(c to 66.0(c? How much heat is required to raise the temperature of 67.0g of water from 25.7(c to 66.0(c? Since specific heat can be. Web do you need a worksheet activity to help gather information about student understanding of specific heat and calorimetry? Web specific heat and calorimetry worksheet key.pdf. A piece of lead (c = 130 j/kg/oc) at 82.0 oc is. Web explain the technique of calorimetry. Web calorimetry problems worksheet 1 note: Specific heat capacityreal examples of specific heatcalorimetry (including parts of a. Web specific heat and calorimetry worksheet a calorimeter has a heat capacity of 4.18 kj/g oc. The initial temperature of the water is 23.00°c. In the table, the first specific heat. Web specific heat and calorimetry (heat lost=heat gained) worksheet 1. Calculate and interpret heat and related properties using typical calorimetry data. G iron rod is heated to a temperature t_1 t 1 and then dropped into 20.\; In this specific heat and calorimetry instructional activity, students are given specific. Web what is the specific heat of the mystery liquid?Awasome Calorimetry Worksheet Answer Key 2023 Alec Worksheet
Specific Heat Worksheet Answer Key
Calculating Specific Heat Worksheet Lovely Calorimetry Worksheets
Calorimetry Worksheet Answer Key
30 Calculating Specific Heat Worksheet Education Template
Specific Heat Worksheet 2 Answer Key Thekidsworksheet
Specific Heat Worksheet Heat Thermodynamic Properties
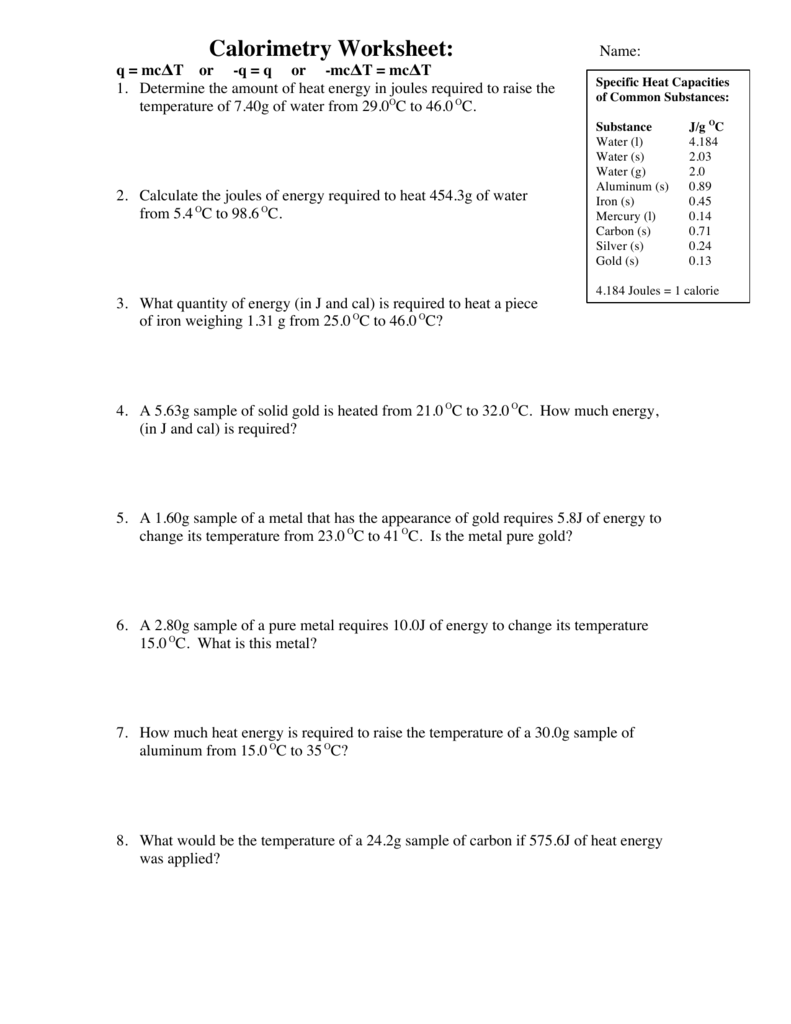
Calorimetry Worksheet
Chapter 6 Calorimetry Worksheet Open the Calorimetry
50 Calorimetry Worksheet Answer Key Chessmuseum Template Library
Web Which Kind Of Substance Needs More Energy To Undergo An Increase Of 5 Oc, Something With A High Or Low Specific Heat?
Web The Specific Heats Of Gases Depend On What Is Maintained Constant During The Heating—Typically Either The Volume Or The Pressure.
G Of Water At A Lower Temperature T_2 T 2 In.
The Specific Heat Capacity Of Water Is 4.184 J/G•°C, And The Specific Heat Capacity Of Mercury Is 0.139 J/G•°C.
Related Post: