Specific Heat And Heat Capacity Worksheet
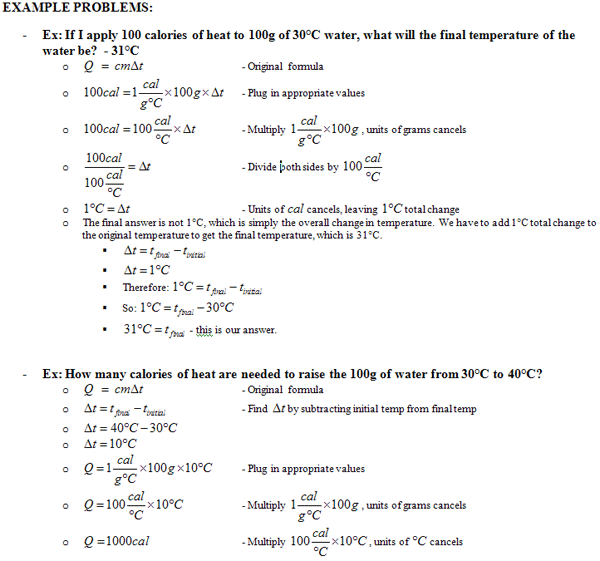
Specific Heat And Heat Capacity Worksheet - Web specific heat capacity worksheet 1. Web 1 find all of the numbers and underline them like this: Cp = q/mδt, where q = heat energy, m = mass, and t = temperature. How much heat did this sample absorb? Examples of how to determine, the heat, heat capacity, and change of temperature. How much heat did this sample absorb? Web specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. Web 2) solve for the heat required to change the water into steam (no change in temp). Calculating specific heat capacity add to my workbooks. Calculate the specific heat capacity of iron. Use q = (m)(δt)(cp) to solve the following problems. Web up to 24% cash back specific heat and heat capacity worksheet the temperature of 335 g of water changed from 24.5oc to 26.4oc. 2.0 kg of carbon is. Web 1 find all of the numbers and underline them like this: Show all work and units. Worksheet/activity file previews docx, 38.38 kb docx, 18.76 kb docx, 19.93 kb doc,. Web specific heat worksheet specific heat directions: How much heat did this sample absorb? Calculate the heat capacity of a piece of ice if 1.30 kg of the wood absorbs 6.75×104 joules of heat, and its temperature changes from 32ºc to 57ºc. Cp = q/mδt, where q. Web specific heat and heat capacity worksheet directions: Web up to 24% cash back specific heat and heat capacity worksheet the temperature of 335 g of water changed from 24.5oc to 26.4oc. Web specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. Cp = q/mδt, where q = heat energy,. 3) calculate the heat required to change the temperature of the steam from 100.0 oc to. How much heat did this sample absorb? How much heat did this sample absorb? Web 2) solve for the heat required to change the water into steam (no change in temp). C for water = 4.18 j/goc. Worksheets are name per work introduction to specific heat capacities, latent heat and specific heat capacity,. Use q = (m)(δt)(cp) to solve the following problems. How much heat did this sample absorb? How much heat did this sample absorb? Web specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. 3) calculate the heat required to change the temperature of the steam from 100.0 oc to. Use q = (m)(δt)(cp) to solve the following problems. Show all work and units. How is knowing the specific heat of an object useful? Calculate the specific heat capacity of iron. Web up to 24% cash back specific heat and heat capacity worksheet the temperature of 335 g of water changed from 24.5oc to 26.4oc. Worksheet/activity file previews docx, 38.38 kb docx, 18.76 kb docx, 19.93 kb doc,. Calculating specific heat capacity add to my workbooks. Web specific heat and heat capacity worksheet directions: Web specific heat capacity worksheet 1. Cp = q/mδt, where q = heat energy, m = mass, and t = temperature. How much heat did this sample absorb? Web practice problems on heat capacity and specific heat. Web specific heat capacity worksheet 1. C for water = 4.18 j/goc. Cp = q/mδt, where q = heat energy, m = mass, and t = temperature. Calculating specific heat capacity add to my workbooks. Web specific heat capacity worksheet. How much heat did this sample absorb? Web 2) solve for the heat required to change the water into steam (no change in temp). Use q = (m)(cp)(at) to solve the following problems. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c °c. Web 1 find all of the numbers and underline them like this: Worksheets are name per work introduction to specific heat capacities, latent heat and specific heat capacity,. Web specific heat and. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c °c. Web 2) solve for the heat required to change the water into steam (no change in temp). 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. Examples of how to determine, the heat, heat capacity, and change of temperature. Web specific heat is closely related to the concept of heat capacity. Worksheet/activity file previews docx, 38.38 kb docx, 18.76 kb docx, 19.93 kb doc,. Cp = q/mδt, where q = heat energy, m = mass, and t = temperature. Web specific heat capacity worksheet 1. Web specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. Calculate the heat capacity of a piece of ice if 1.30 kg of the wood absorbs 6.75×104 joules of heat, and its temperature changes from 32ºc to 57ºc. How much heat did this sample absorb? Web practice problems on heat capacity and specific heat. Worksheets are name per work introduction to specific heat capacities, latent heat and specific heat capacity,. Show all work and units. Show all work and units q = heat transfer (cal) m = mass (g) cp. C for water = 4.18 j/goc. Web specific heat capacity subject: Use q = (m)(δt)(cp) to solve the following problems. Web specific heat capacity worksheet. How is knowing the specific heat of an object useful? Worksheet/activity file previews docx, 38.38 kb docx, 18.76 kb docx, 19.93 kb doc,. C for water = 4.18 j/goc. Web specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. Web up to $3 cash back specific heat and heat capacity worksheet. How much heat did this sample absorb? How much heat did this sample absorb? Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c °c. Show all work and units q = heat transfer (cal) m = mass (g) cp. Worksheets are name per work introduction to specific heat capacities, latent heat and specific heat capacity,. A block of aluminium has a specific heat capacity of 900 j/kg o c and is heated from a starting temperature of 0 to a final. 3) calculate the heat required to change the temperature of the steam from 100.0 oc to. Calculating specific heat capacity add to my workbooks. Explain what the specific heat of an object means/tells us? Web specific heat is closely related to the concept of heat capacity. Calculate the heat capacity of a piece of ice if 1.30 kg of the wood absorbs 6.75×104 joules of heat, and its temperature changes from 32ºc to 57ºc. How is knowing the specific heat of an object useful?12 Best Images of Heat Worksheet 1 Specific Heat Calculations
Specific Heat Worksheet Answer Key —
Specific Heat Capacity Worksheet DocsLib
42 specific heat capacity worksheet answers Worksheet Master
Specific Heat and Heat Capacity Worksheet
specific heat chemistry worksheet
PWHS Thermodynamics Specific Heat Worksheet
14 Best Images of And Specific Heat Capacity Worksheet Specific Heat
Specific Heat Worksheet Answer Key
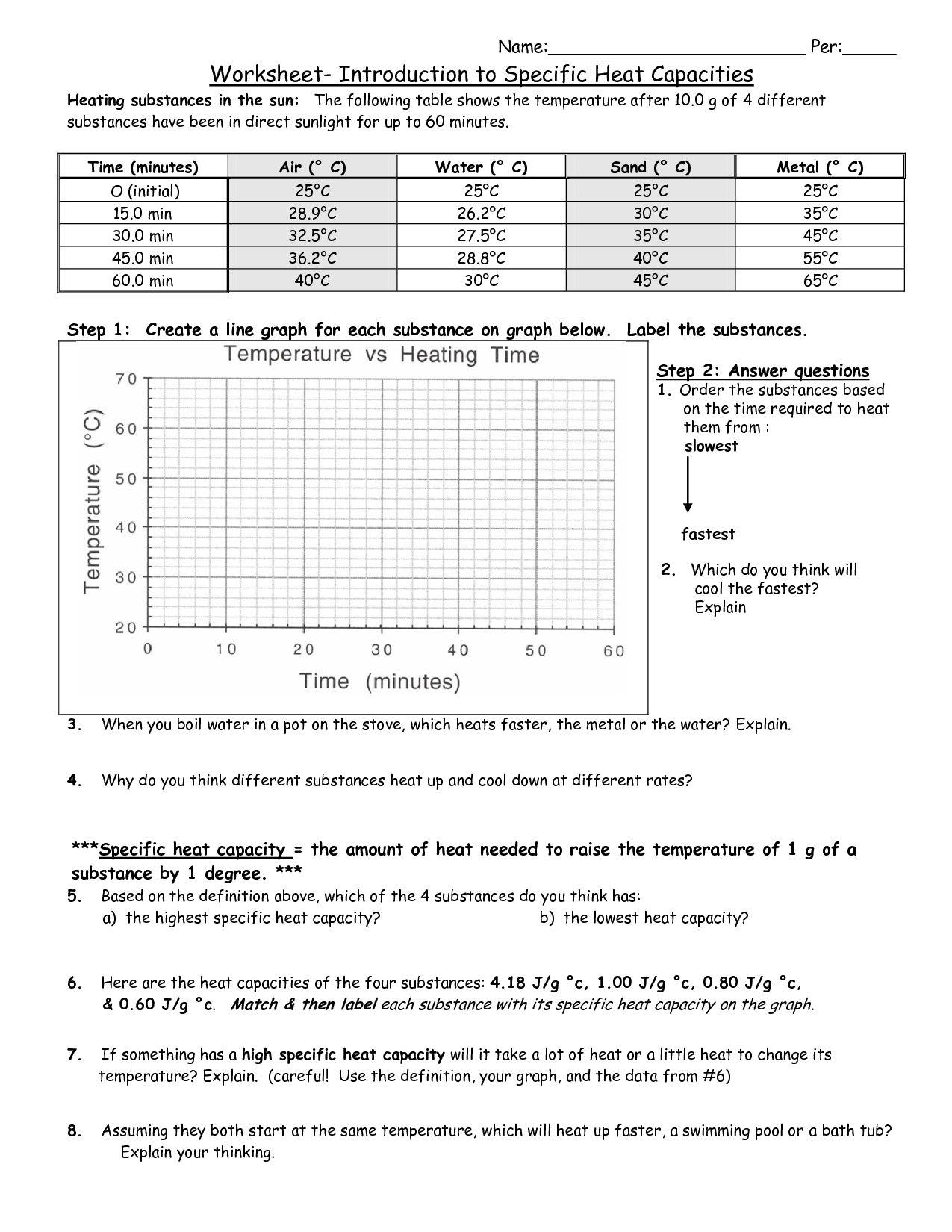
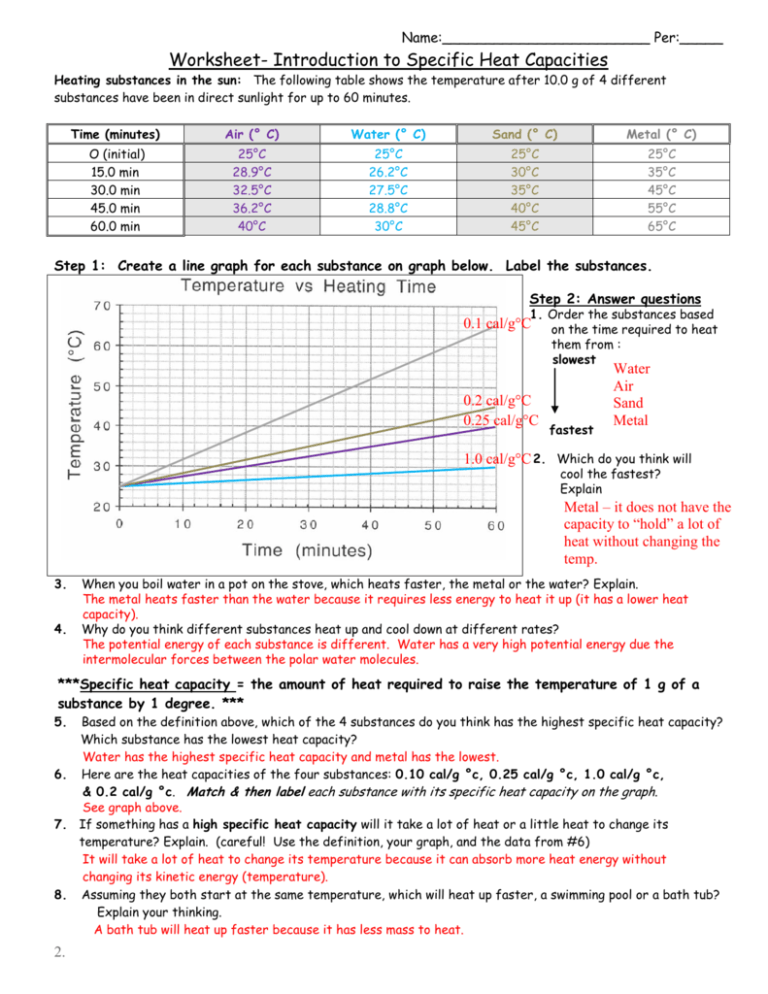
Worksheet Introduction to Specific Heat Capacities
Web 1 Find All Of The Numbers And Underline Them Like This:
Use Q = (M)(Δt)(Cp) To Solve The Following Problems.
2.0 Kg Of Carbon Is.
Web Up To 24% Cash Back Specific Heat And Heat Capacity Worksheet The Temperature Of 335 G Of Water Changed From 24.5Oc To 26.4Oc.
Related Post: