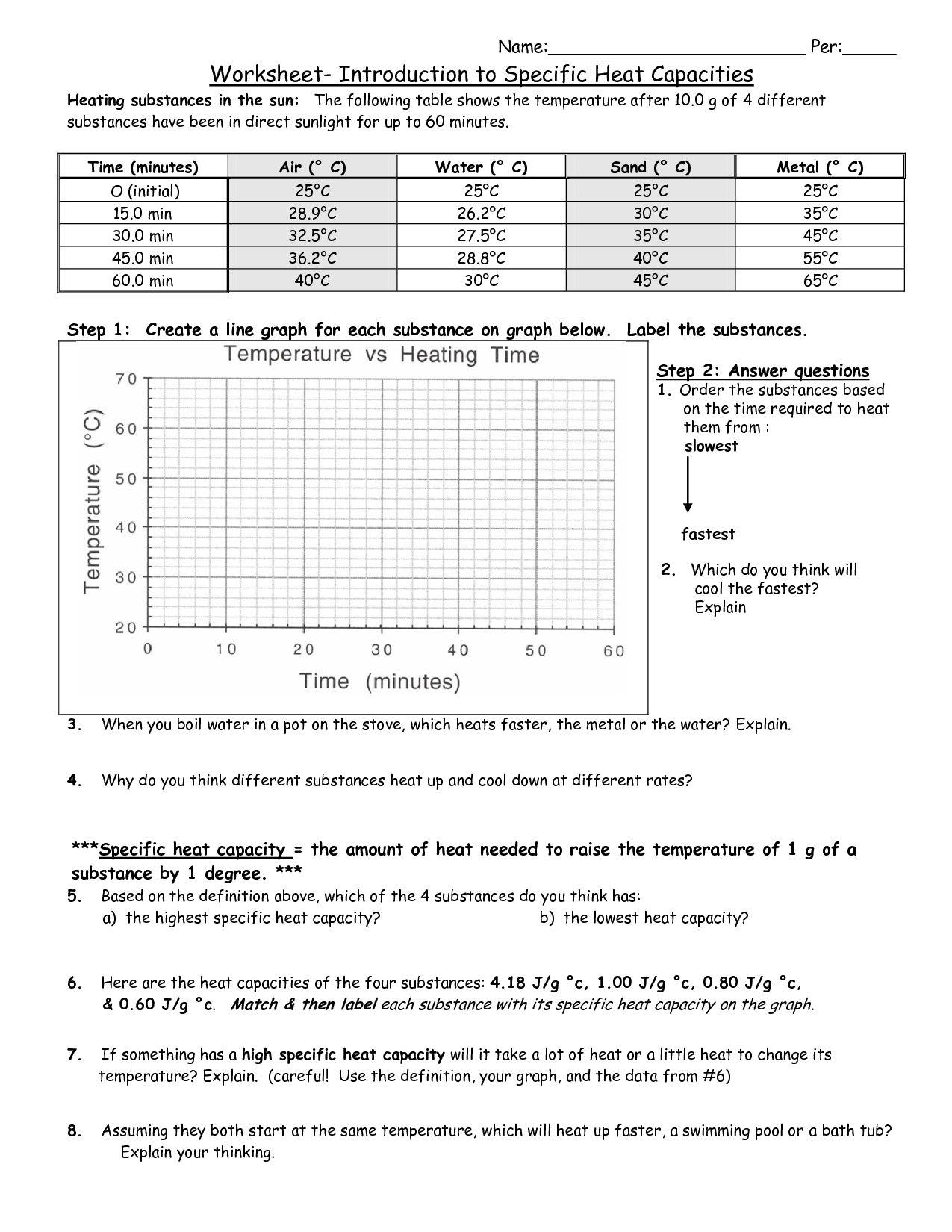
Specific Heat Practice Worksheet
Specific Heat Practice Worksheet - Use q = (m)(cp))(δt) to solve the following problems. All questions ask students to solve for q in accordance with massachusetts state frameworks in physics and so students do not need to know the algebra required to rearrange the equation. Collection of tes resources with presentation. Suppose the skillet is cooled to room temperature, 23.9 oc. Use q = (m)(δt)(cp) to solve the following problems. Web in this problem set, students will be utilizing data to solve specific heat equation problems and identify if the system is gaining or losing heat. Calculate the total amount of heat a beaker of water would absorb sitting in the sun. 25 g of mercury is heated from 25°c to 155°c, and absorbs 455 joules of heat in the process. Calculate the heat gained by 125.0 g of water when it is put into a calorimeter and its Calculate the specific heat capacity of iron. Use q = (m)(δt)(cp) to solve the following problems. (specific heat capacity of granite is 0.1 cal/gºc). Remember, the specific heat is the quantity of heat energy necessary to increase the temperature of 1 g of a substance by 1°c. Answers and solutions are included. Web this worksheet includes questions that ask students to solve for heat using the heat. M c t c m t. 8 x 10 2 cal to calories c. Collection of tes resources with presentation. A list of specific heats can be found in your chemistry book. Identify each variables by name & the units associated with it. Concepts:students will practice calculating heat (q) using the equation q = mc∆t. Explain how they differ from each other. 8 x 10 2 cal to calories c. Q m c t t m c. How much heat is absorbed by 20g granite boulder as energy from the sun causes its temperature to change from 10°c to 29°c? How much heat is needed to raise a 0.30 kg piece of aluminum from 30.(c to 150(c? Web calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes from 32°c to 57°c. Web this worksheet includes questions that ask students to solve for heat using. Concepts:students will practice calculating heat (q) using the equation q = mc∆t. Q = heat absorbed m = mass δt = change in temperature c = of material. Web specific heat capacity | teaching resources. Web using heat energy and specific heat calculations, students will determine the specific heat of an unknown metal (evidence from a crime scene) and use. If the temperature of 100 g of water increases from 10 to 45 c, how much heat is absorbed? A list of specific heats can be found in your chemistry book. Identify each variables by name & the units associated with it. 5.0 g of copper was heated from 20°c to 80°c. Q=mcδt (specific heat of water= 4 j/g°c or. How much heat in kilojoules is released when 25.0 g of water is cooled from 85.0ºc to 40.0ºc? What is the specific heat capacity of silver metal if 55 g of the metal absorbs 47. ⭐editable powerpoint presentation for students & The water has a mass of 500g, a specific heat of 4.184 (j/gx0c), and had a change in temperature. How much energy must be absorbed by 20 g of water to. Answers and solutions are included. Use as a homework, bellringer, exit slip, supplemental practice, or quiz. Q=mcδt (specific heat of water= 4 j/g°c or 1 cal/g°c) 1. (q= m c δ t) b. A metal weighing 50.0 g absorbs 220.0 j of heat when its temperature increases by 120.0°c. Web calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes from 32°c to 57°c. (specific heat capacity of cu is 0.092 cal/g °c). Web students will calculate the. Web specific heat worksheet 1. ⭐editable powerpoint presentation for students & Answers and solutions are included. All questions ask students to solve for q in accordance with massachusetts state frameworks in physics and so students do not need to know the algebra required to rearrange the equation. Calculate the specific heat capacity of iron. Web students will calculate the unknown variables of mass, energy, temperature, and specific heat. Q=mcδt (specific heat of water= 4 j/g°c or 1 cal/g°c) 1. How much heat in kilojoules is released when 25.0 g of water is cooled from 85.0ºc to 40.0ºc? Heat is not the same as temperature, yet they are related. This is an intensive physical property and has been calculated for all common substances. Web specific heat capacity | teaching resources. Water 4.179 aluminum 0.900 copper 0.385 iron 0.450 granite 0.790. Calculate the heat gained by 125.0 g of water when it is put into a calorimeter and its Calculate the specific heat capacity of iron. Web calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes from 32°c to 57°c. Show all work and proper units. If the temperature of 100 g of water increases from 10 to 45 c, how much heat is absorbed? A table of specific heat values is provided on page 2 of this worksheet. Concepts:students will practice calculating heat (q) using the equation q = mc∆t. A list of specific heats can be found in your chemistry book. Web using heat energy and specific heat calculations, students will determine the specific heat of an unknown metal (evidence from a crime scene) and use this calculation to solve the case of the unknown metal. Use q = (m)(cp))(δt) to solve the following problems. 5 kj to cal 2. Calculate the specific heat capacity of mercury. How much energy must be absorbed by 20 g of water to. Web specific heat worksheet 1. How much energy must be absorbed by 20 g of water to. Forms allow for quick auto grading and instant data feedback for you and your students. How many joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22°c to 55°c, if the specific heat of aluminum is 0.90 j/g°c? How much heat is needed to raise a 0.30 kg piece of aluminum from 30.(c to 150(c? Heat is not the same as temperature, yet they are related. C = t/m ̈t, zheue t = heaw eneug, m = mavv, and t = wempeuawxue remembeu, ̈t = (tfinal ± tinitial). How much heat in kilojoules is released when 25.0 g of water is cooled from 85.0ºc to 40.0ºc? Suppose the skillet is cooled to room temperature, 23.9 oc. How much energy, in the form of heat, is lost when a 453 g piece of lead cools from 276 ºc to 2 ºc? (specific heat capacity of cu is 0.092 cal/g °c). Web this worksheet includes questions that ask students to solve for heat using the heat equation, q=mct. Web q = m x ∆t x c. Explain how they differ from each other. A list of specific heats can be found in your chemistry book. Q = heat absorbed m = mass δt = change in temperature c = of material.14 Best Images of And Specific Heat Capacity Worksheet Specific Heat
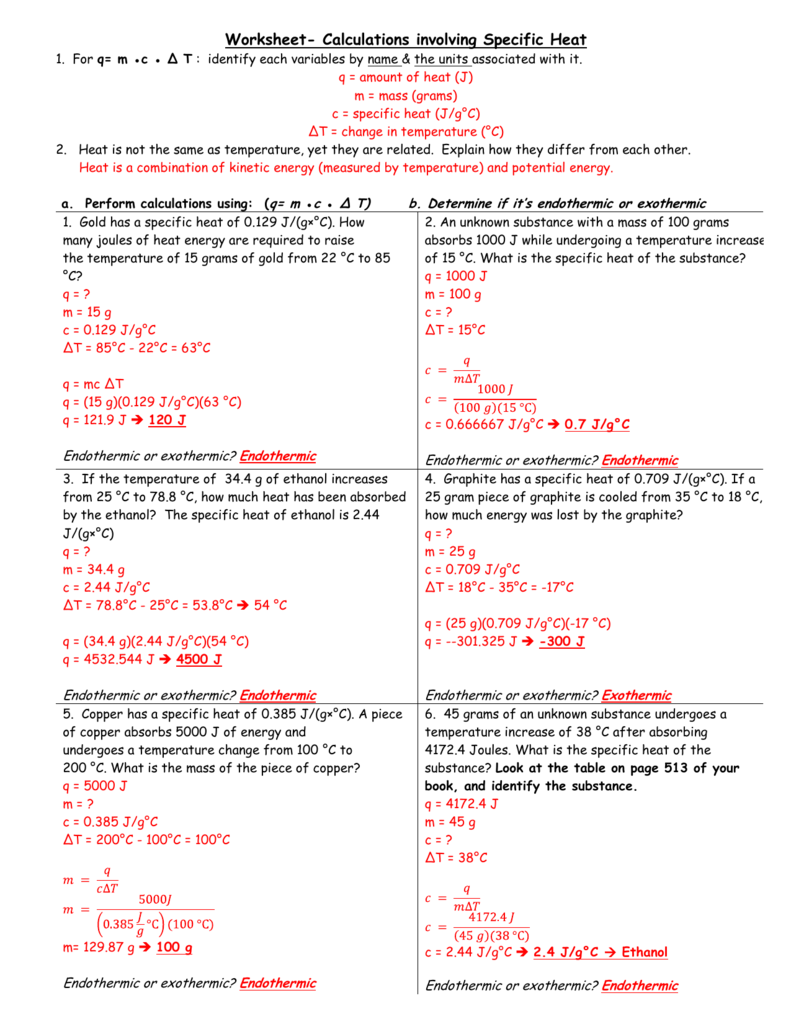
Worksheet Calculations Involving Specific Heat
Calculating Specific Heat Worksheet
Specific Heat Practice Worksheet —
Specific Heat Chem Worksheet 16 1 Answer Key —
Specific Heat Worksheet 2 Answer Key Thekidsworksheet
Specific Heat Practice Problems Specific Heat Worksheet C = T/m ̈T
Specific Heat Practice Worksheet —
25 Worksheet Calculations Involving Specific Heat Answer Key support
40 specific heat capacity worksheet answers Worksheet Resource
An Aluminum Skillet Weighing 1.58 Kg Is Heated On A Stove To 173 Oc.
Use Q = (M)(Cp))(Δt) To Solve The Following Problems.
How Much Energy, In The Form Of Heat, Is Needed To Change The Temperature Of A Copper Bracelet (30 G) From25 ºc To 86 ºc?
Calculate The Total Amount Of Heat A Beaker Of Water Would Absorb Sitting In The Sun.
Related Post: