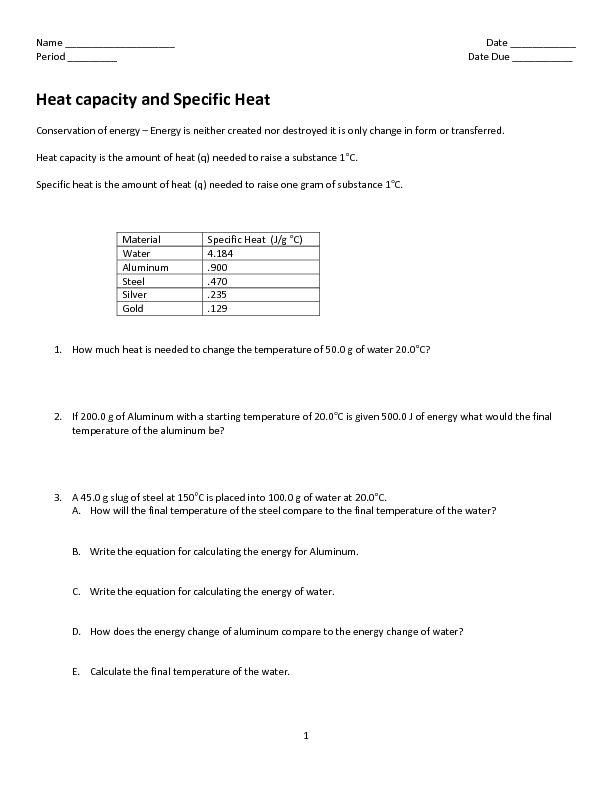
Specific Heat Worksheet Answers
Specific Heat Worksheet Answers - Web in this simulation, you solved for the specific heat capacity of several materials, but the specific heat for many materials is known. How many joules of heat are. Web know the first law of thermodynamics. This problem requires several steps since temperature Worksheet (50 mixed questions, including 10 challenging. How much heat is needed to raise the temperature of 50.0 g of water by 25.0°c 2. Web chandler unified school district How much heat is released? The table to the right list the specific heat (heat capacity) of four common materials aluminum, beryllium, gold, and. Understand hess’s law and its use for calculating reaction enthalpies. This problem requires several steps since temperature How much energy must be a bsorbed by 20.0 g. Show all work and proper units. How much heat is released? 50 assorted questions answers included. 50 assorted questions answers included. Web answers = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Understand hess’s law and its use for calculating reaction enthalpies. Web suitable for gcse and a level physics. Web specific heat and heat capacity worksheet directions: How much heat is necessary to change a 52.0 g sample of water at 33.0oc into steam at 110.0 oc? Web specific heat capacity the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Use q = (m)(cp))(δt) to solve the following problems. Web ch 16.1 specific heat. Web suitable for gcse and a level physics. Web specific heat capacity the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Web know the first law of thermodynamics. Understand hess’s law and its use for calculating reaction enthalpies. This problem requires several steps since temperature Worksheet/activity file previews docx, 38.38 kb docx, 18.76 kb docx, 19.93 kb doc,. Web ch 16.1 specific heat pr q=mcδt (specific heat of water= 4.184 j/g°c or 1.00 cal/g°c) 444 cal to joules b. How much heat is absorbed by a 600 g iron. Web know the first law of thermodynamics. How much heat is necessary to change a 52.0. The specific heat capacity is the amount of heat energy required to raise the temperature of a system with a mass of m m by \delta t δt so applying its. How much heat is absorbed by a 600 g iron. Specific heat table on the back 1. Web specific heat and heat capacity worksheet directions: How much heat is. Web specific heat capacity the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Web chandler unified school district The specific heat of iron = 0.450 j/g °c. Use q = (m)(cp))(δt) to solve the following problems. Show all work and proper units. How much heat is absorbed by a 600 g iron. How many joules of heat are. Answer 15.6 kj solution a similar question to that in problem 8, and we are going to. Web suitable for gcse and a level physics. Show all work and proper units. So, we can now compare the specific heat capacity of a substance on a per gram bases. Web what is the specific heat of the sample? Show all work and units. Understand the principles of calorimetry. Use q = (m)(cp))(δt) to solve the following problems. Answer 15.6 kj solution a similar question to that in problem 8, and we are going to. How many joules of heat are. C = t/m ̈t, zheue t = heaw eneug, m = mavv, and t = wempeuawxue remembeu, ̈t = (tfinal ± tinitial). Show all work and units. Understand the relationships between heat, work, internal energy, and enthalpy. Answer 15.6 kj solution a similar question to that in problem 8, and we are going to. Worksheet/activity file previews docx, 38.38 kb docx, 18.76 kb docx, 19.93 kb doc,. Use q = (m)(cp))(δt) to solve the following problems. Web specific heat and heat capacity worksheet directions: Worksheet (50 mixed questions, including 10 challenging. Web chandler unified school district Web suitable for gcse and a level physics. Understand hess’s law and its use for calculating reaction enthalpies. The specific heat capacity is the amount of heat energy required to raise the temperature of a system with a mass of m m by \delta t δt so applying its. Web ch 16.1 specific heat pr q=mcδt (specific heat of water= 4.184 j/g°c or 1.00 cal/g°c) 444 cal to joules b. How much heat is absorbed by a 600 g iron. So, we can now compare the specific heat capacity of a substance on a per gram bases. How many joules of heat are. This value also depends on the nature of the chemical bonds in the substance, and its phase. Web specific heat capacity subject: Web what is the specific heat of the sample? Web answers = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Understand the principles of calorimetry. Web specific heat capacity the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. 50 assorted questions answers included. Worksheet (50 mixed questions, including 10 challenging. This high school chemistry inquiry activity walks students through a. Understand hess’s law and its use for calculating reaction enthalpies. Web ch 16.1 specific heat pr q=mcδt (specific heat of water= 4.184 j/g°c or 1.00 cal/g°c) 444 cal to joules b. Answer 15.6 kj solution a similar question to that in problem 8, and we are going to. Web specific heat capacity the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Specific heat table on the back 1. Web specific heat capacity subject: This value also depends on the nature of the chemical bonds in the substance, and its phase. Web in this simulation, you solved for the specific heat capacity of several materials, but the specific heat for many materials is known. Understand the concepts of heat capacity, molar heat capacity, and specific heat. How much heat is necessary to change a 52.0 g sample of water at 33.0oc into steam at 110.0 oc? How much heat is absorbed by a 600 g iron. Understand the relationships between heat, work, internal energy, and enthalpy. Web answers = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. In a household radiator, 1000.g of steam at 100.Worksheet Calculations Involving Specific Heat
30 Specific Heat Worksheet Answer Key Education Template
heat answers DriverLayer Search Engine
Specific Heat Worksheet Answers
Specific Heat Answers 2013 Heat Capacity
30 Specific Heat Worksheet Answer Key Education Template
Specific Heat Worksheet Answer Key
Specific Heat Worksheet 2 Answer Key Kidsworksheetfun
Specific Heat Worksheet Answers
Specific Heat Worksheet Answers
Use Q = (M)(Cp))(Δt) To Solve The Following Problems.
Web Specific Heat And Heat Capacity Worksheet Directions:
How Much Heat Is Needed To Raise The Temperature Of 50.0 G Of Water By 25.0°C 2.
How Much Heat Is Released?
Related Post: