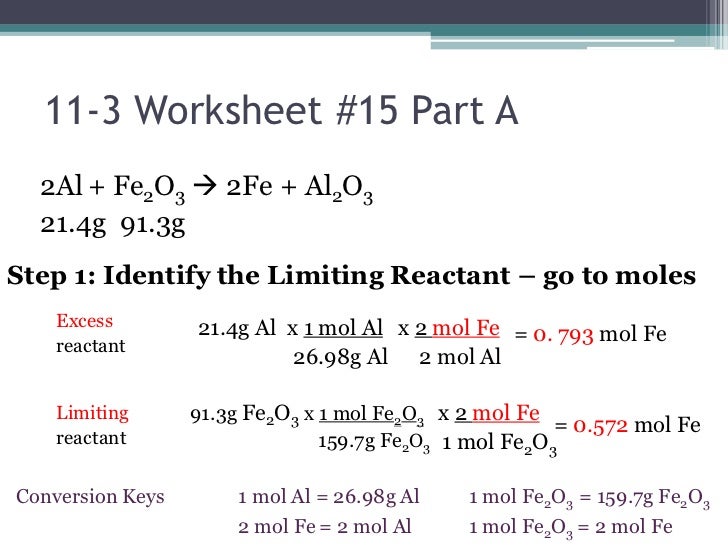
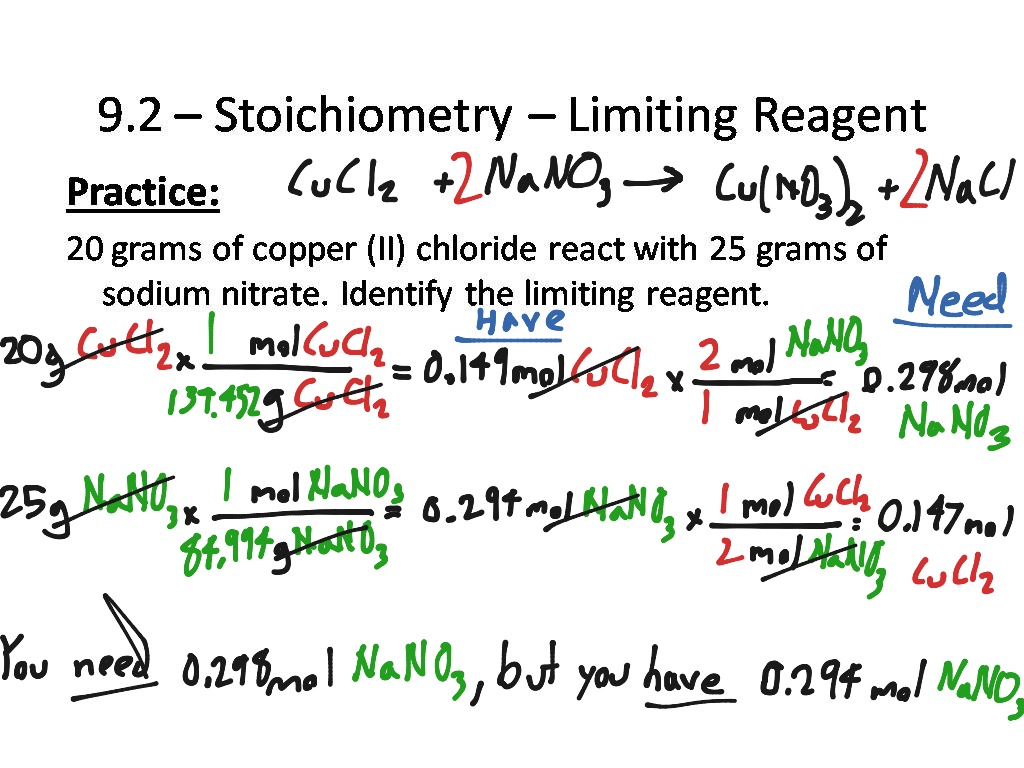Stoichiometry Limiting Reagent Worksheet Answers
Stoichiometry Limiting Reagent Worksheet Answers - Web using your knowledge of stoichiometry and limiting reagents, answer the following questions: Web oxygen on hand ⇒ 10.0 g / 31.9988 g/mol = 0.3125 mol. 15 how much of the excess reagent will be left over after the reaction is. 2015 ap chemistry free response 2a (part 1 of 2) We are now ready to pull everything we know about reaction stoichiometry together, and answer the question: Web understand how the mole concept is applied to determining empirical formulas from analytical data. You have 0.20 mol of \(ca(oh)_2\) and you need 0.17 mol \(ca(oh)_2\). There’s no end to what you can achieve… unless there’s a limiting reagent involved. Web this online quiz is intended to give you extra practice in performing stoichiometric conversions, including limiting reagent and percent yield problems. A) write the balanced equation for the reaction given above: Given some initial amount of reactants, what should be present after a chemical reaction goes to completion? Web if you have more than you need, this is the reagent in excess (xs). 2mg + o 2 → 2mgo. Web this online quiz is intended to give you extra practice in performing stoichiometric conversions, including limiting reagent and percent yield problems.. If you have less than you need, this is the limiting reagent (lr). You have 0.17 mol of \(h_2so_4\) and you need 0.20 mol \(h_2so_4\). Web 50 stoichiometry worksheet answer key. If given mass, divide by formula weight to convert to moles (this is the mass to mole step from the section 4.1,3. It will be based on the mass. Calculating the amount of product formed from a limiting reactant. Web understand how the mole concept is applied to determining empirical formulas from analytical data. Web to purchase homework worksheets only: You have 0.17 mol of \(h_2so_4\) and you need 0.20 mol \(h_2so_4\). Web if you have more than you need, this is the reagent in excess (xs). Limiting reactant and reaction yields. If given mass, divide by formula weight to convert to moles (this is the mass to mole step from the section 4.1,3. Web stoichiometry/limiting reagent practice ap chemistry (practice, practice, practice. Web the principles of stoichiometry and limiting reagents will be used to predict the amount of product that should be produced when mixing two. The smaller of these two answers is correct, and the reagent that leads to this answer is the limiting reagent. Understand how the mole concept allows prediction of the mass relationships between reactants and products in a chemical reaction. 2015 ap chemistry free response 2a (part 1 of 2) You have 0.17 mol of \(h_2so_4\) and you need 0.20 mol. Web 50 stoichiometry worksheet answer key. Web if you have more than you need, this is the reagent in excess (xs). Automotive airbags inflate when sodium azide, nan 3 , rapidly decomposes to its component elements: Get your pv = nrt mixed with your stoich! A) write the balanced equation for the reaction given above: This reagent is the lr You have 0.20 mol of \(ca(oh)_2\) and you need 0.17 mol \(ca(oh)_2\). Web 50 stoichiometry worksheet answer key. Understand the concept of the limiting reagent. Web this online quiz is intended to give you extra practice in performing stoichiometric conversions, including limiting reagent and percent yield problems. If 6.80 g of ph. 1 cucl2 b) c) d) e) f) + 2 nano3 1 cu (no3)2 + 2 nacl. Great labs/activities that reinforce these concepts: If you have less than you need, this is the limiting reagent (lr). Since the oxygen required is greater than that on hand, it will run out before the sucrose. Web using your knowledge of stoichiometry and limiting reagents, answer the following questions: Great labs/activities that reinforce these concepts: Web limiting reagent calculations are performed in the same manner as the stoichiometric equations on worksheet #11. You have 0.17 mol of \(h_2so_4\) and you need 0.20 mol \(h_2so_4\). Gravimetric analysis and precipitation gravimetry. April 24, 2019 by chess93. You have 0.20 mol of \(ca(oh)_2\) and you need 0.17 mol \(ca(oh)_2\). Divide moles of each reactant by it's stoichiometric coefficient. 1) write the balanced equation for the reaction of lead (ii) nitrate with sodium iodide to form sodium nitrate and lead (ii) iodide: This reagent is the lr .show all work (including balanced equations). Web stoichiometry/limiting reagent practice ap chemistry (practice, practice, practice. Therefore, mg is the limiting reagent. Web understand how the mole concept is applied to determining empirical formulas from analytical data. We are now ready to pull everything we know about reaction stoichiometry together, and answer the question: Oxygen is the limiting reagent. Web using your knowledge of stoichiometry and limiting reagents, answer the following questions: 1) write the balanced equation for the reaction of lead (ii) nitrate with sodium iodide to form sodium nitrate and lead (ii) iodide: Identify the limiting reactant when 4.687 g This reagent is the lr Limiting reactant and reaction yields. Calculating the amount of product formed from a limiting reactant. 15 how much of the excess reagent will be left over after the reaction is. (a) write a balanced molecular equation for the reaction of hydrochloric acid with sodium hydroxide. Web if you have more than you need, this is the reagent in excess (xs). Select your preferences below and click 'start' to. Web 50 stoichiometry worksheet answer key. For 4.7 moles of o 2 = (2moles of mg / 1 mole of o 2) × 4.7 moles o 2 = 9.4 moles mg. Web limiting reagent calculations are performed in the same manner as the stoichiometric equations on worksheet #11. Web oxygen on hand ⇒ 10.0 g / 31.9988 g/mol = 0.3125 mol. Understand the concept of the limiting reagent. This reagent is in xs. Web gas stoichiometry worksheet: The smaller of these two answers is correct, and the reagent that leads to this answer is the limiting reagent. It will be based on the mass of the reactants present, and on the stoichiometry of the reaction. Web oxygen on hand ⇒ 10.0 g / 31.9988 g/mol = 0.3125 mol. Select your preferences below and click 'start' to. 2mg + o 2 → 2mgo. Web if you have more than you need, this is the reagent in excess (xs). We are now ready to pull everything we know about reaction stoichiometry together, and answer the question: Web which is the limiting reagent? However, with a limiting reagent, you must calculate the amount of product obtained from each reactant (that means doing math/stoichiometry at. Web stoichiometry/limiting reagent practice ap chemistry (practice, practice, practice. Great labs/activities that reinforce these concepts: You have 0.20 mol of \(ca(oh)_2\) and you need 0.17 mol \(ca(oh)_2\). Web this online quiz is intended to give you extra practice in performing stoichiometric conversions, including limiting reagent and percent yield problems.Stoichiometry Limiting Reagent Worksheet Answers Amwintersun
Limiting Reagent Worksheet Answers Briefencounters
Stoichiometry Limiting Reagent Worksheet Answers —
Stoichiometry Limiting Reagent Worksheet
stoichiometry worksheet limiting reagent
Balancing Chemical Equations Quizmo Answers Chemistry Balancing
Gas Stoichiometry Worksheet Answer Key
Stoichiometry Limiting Reagent Worksheets Answers
Stoichiometry Limiting Reagent Worksheet
Problem Sheet 3 Stoichiometry Limiting Reactants
Gravimetric Analysis And Precipitation Gravimetry.
62269 (Obtain From Video) Mass Of Evaporating Dish And Nacl 55.8950 G Mass Of.
Limiting Reactant Stoichiometry Worksheet Ii Answer Key 11 From Stoichiometry Worksheet Answer Key , Image Source:
Web Limiting Reactants And Solution Stoichiometry.
Related Post: