Stoichiometry Mole Mole Problems Worksheet Answers
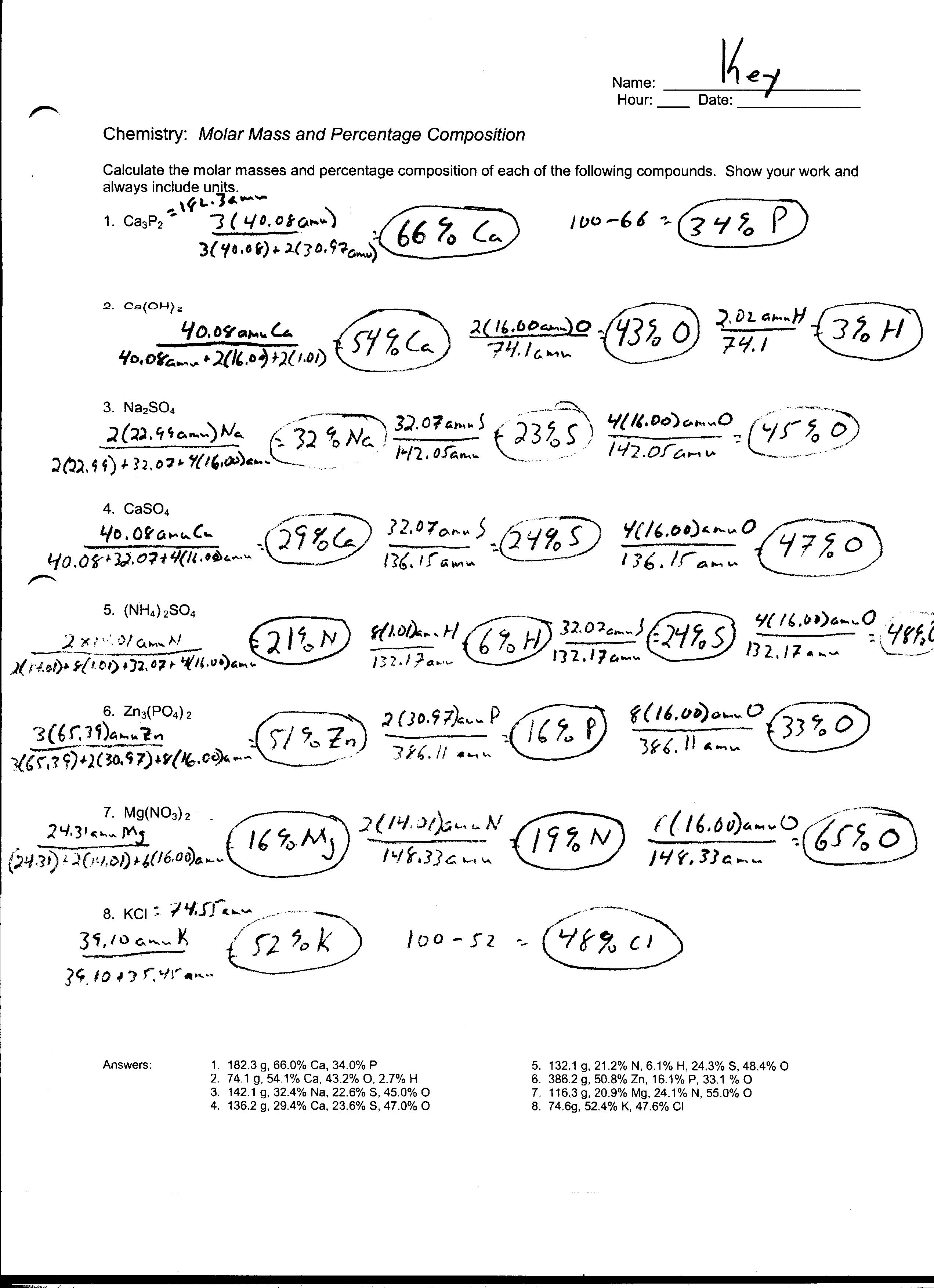
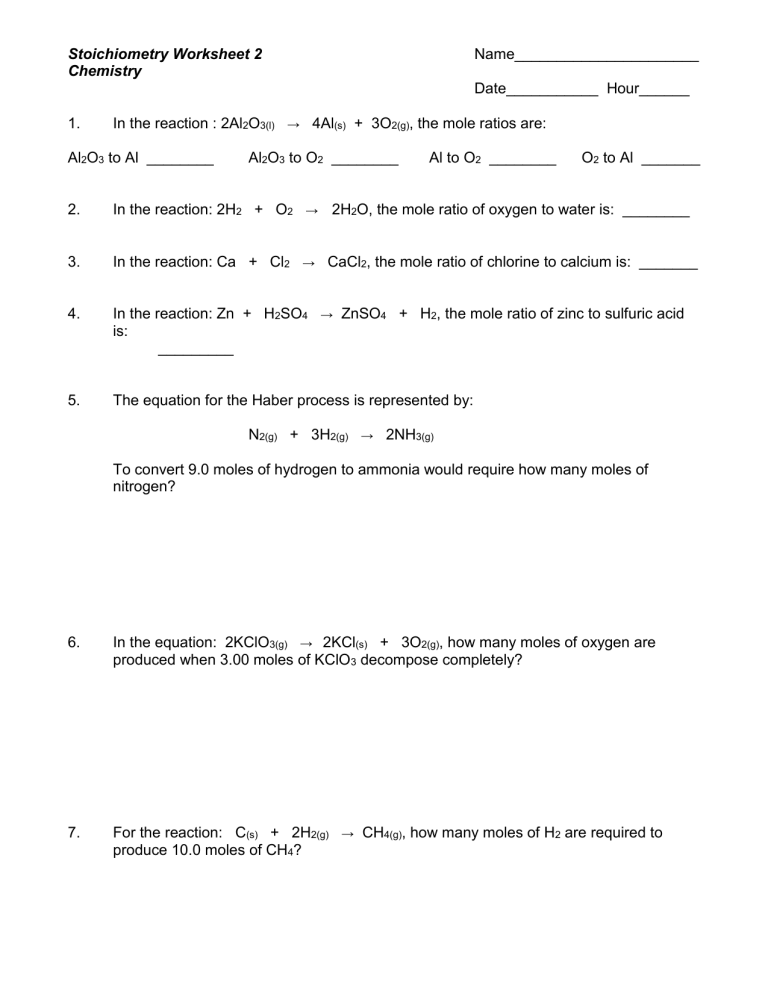
Stoichiometry Mole Mole Problems Worksheet Answers - A detailed answer key is included. Grams of a is converted to moles by multiplying by the inverse of the molar mass. Using mole and mass relationships, we can calculate the mass of product that should be produced from a given amount of reactant when it is completely consumed in the reaction. I l i l | stoichiometry: When 80 grams of aluminum is reacted with excess chlorine gas, how many formula units of alcl3 are produced? 1.077 moles of magnesium phosphate 5. The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass of 12.0107 g. Web worksheet for basic stoichiometry part 1: Web flowchart of steps in stoichiometric calculations. If you know the number of moles of 1 substance, the balanced equation. The atomic weight refers to the weighted average of masses of the isotopes comprising a naturally occurring sample of carbon. A detailed answer key is included. Mole/mole and mole/mass stoichiometry problems. Reactants and products are related by a fixed ratio. Each worksheet features 7 unique one, two, and three step stoichiometry problems including moles to mass, mole to mole, volume. Web flowchart of steps in stoichiometric calculations. In this type of problem, the mass of one substance is given, usually in grams. What is the mass of 2 moles of h2s ? Grams of a is converted to moles by multiplying by the inverse of the molar mass. Perfect for classwork, homework, extra practice, or examples for students in a. Directions you must solve each of the following problems using dimensional analysis. What is the mass of 2 moles of h2s ? If you know the number of moles of 1 substance, the balanced equation. 1.077 moles of magnesium phosphate 5. 182g hcl what is the mass in grams of h2 gas when 4.0 moles of hcl is added to. The mole and avogadro's number. 335.55 x 10 molecules (1 𝑜 23 𝑜 𝑒𝑐 𝑒 ) = 9.22 x 109 moles h 2 so 4 5. One mole of anything is 6.02 x 10 23, so it is 6.02 x 10 mg atoms. Directions you must solve each of the following problems using dimensional analysis. How many grams of kcl. How many moles of o2 can be produced by letting 12.00 moles of kclo3 react? Perfect for classwork, homework, extra practice, or examples for students in a distance learning setting. Web this is a series of lectures in videos covering chemistry topics taught in high schools. Using mole and mass relationships, we can calculate the mass of product that should. 40.08 grams of calcium is one mole (see periodic table), and one mole is 6.02 x 1023 atoms. I l i l | stoichiometry: Web flowchart of steps in stoichiometric calculations. When 80 grams of aluminum is reacted with excess chlorine gas, how many formula units of alcl3 are produced? Mole/mole and mole/mass stoichiometry problems. Using mole and mass relationships, we can calculate the mass of product that should be produced from a given amount of reactant when it is completely consumed in the reaction. ×34 grams = 68 grams. The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass of 12.0107 g. Hydrogen gas can be produced through. Web key questions & exercises 1. Mg(s) + 2hcl(aq) ( mgcl2(aq) + h2(g) how many grams of hcl are consumed by the reaction of 2.50 moles of magnesium? Gfm of s = 32>br> gfm of h2s = 2×1 + 32 = 34 grams / mole. One mole of anything is 6.02 x 10 23, so it is 6.02 x 10. When 80 grams of aluminum is reacted with excess chlorine gas, how many formula units of alcl3 are produced? From this, you are to determine the amount in moles of another substance that will either react with or be produced from the given substance. Directions you must solve each of the following problems using dimensional analysis. Hydrogen gas can be. How many grams of kcl is produced from 1.00 g of cl2 and excess k ? What is the mass of 2 moles of h2s ? Based on the following equation, how many moles of each product are produced when 5.9 moles of zn(oh)2 are reacted with h3po4? Reactants and products are related by a fixed ratio. Grams of a. 1.077 moles of magnesium phosphate 5. They will be given the moles of a product or reactant, then use molar (coefficient) ratios to convert. How many moles of o2 can be produced by letting 12.00 moles of kclo3 react? Mole ←→ mass conversions convert the following number of moles of chemical into its corresponding mass in grams. During the lesson, watch and listen for instructions to take notes, pause the video, complete an. Add to my workbooks (15) A detailed answer key is included. When 80 grams of aluminum is reacted with excess chlorine gas, how many formula units of alcl3 are produced? Hydrogen gas can be produced through the following reaction. Web mass to moles problems. 0.031 moles of aluminum iodide 4. 0.436 moles of ammonium chloride 2. 0.50 moles of calcium nitrate Reactants and products are related by a fixed ratio. Mass of given → moles of given → moles of unknown. Perfect for classwork, homework, extra practice, or examples for students in a distance learning setting. 2.360 moles of lead (ii) oxide 3. Web this is a series of lectures in videos covering chemistry topics taught in high schools. 335.55 x 10 molecules (1 𝑜 23 𝑜 𝑒𝑐 𝑒 ) = 9.22 x 109 moles h 2 so 4 5. N2 + 3h2 > 2nh3 how many moles If you know the number of moles of 1 substance, the balanced equation. From this, you are to determine the amount in moles of another substance that will either react with or be produced from the given substance. 0.50 moles of calcium nitrate Web worksheet for basic stoichiometry part 1: Mole/mole and mole/mass stoichiometry problems. 40.08 grams of calcium is one mole (see periodic table), and one mole is 6.02 x 1023 atoms. 0.436 moles of ammonium chloride 2. How many grams of kcl is produced from 2.50 g of k and excess cl2 ? The mole and avogadro's number. Web flowchart of steps in stoichiometric calculations. Reactants and products are related by a fixed ratio. Moles of a is converted to moles of b by multiplying by the molar ratio. N2 + 3h2 > 2nh3 how many moles Based on the following equation, how many moles of each product are produced when 5.9 moles of zn(oh)2 are reacted with h3po4? Mole ←→ mass conversions convert the following number of moles of chemical into its corresponding mass in grams. The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass of 12.0107 g.15 Chemistry Mole Worksheet /
Mole Mole Stoichiometry Worksheet Answers qualityinspire
5 Best Images of Chemistry If8766 Worksheet Answer Key Chemistry Word
Stoichiometry Mixed Problems Chemistry If8766 Answers MIXERXC
Stoichiometry Calculation Practice Worksheet
Stoichiometry Worksheet 2 (1)
19+ Stoichiometry Practice Worksheet 2 Answers incognosis
FREE 9+ Sample Stoichiometry Worksheet Templates in MS Word PDF
Stoichiometry Worksheet+Answers Mole (Unit) Iron
Gas Stoichiometry Worksheet Answer Key
The Atomic Weight Refers To The Weighted Average Of Masses Of The Isotopes Comprising A Naturally Occurring Sample Of Carbon.
Circle The Final Answer, Giving Units And The Correct Number Of Significant Figures.
Web Mass To Moles Problems.
Grams Of A Is Converted To Moles By Multiplying By The Inverse Of The Molar Mass.
Related Post: