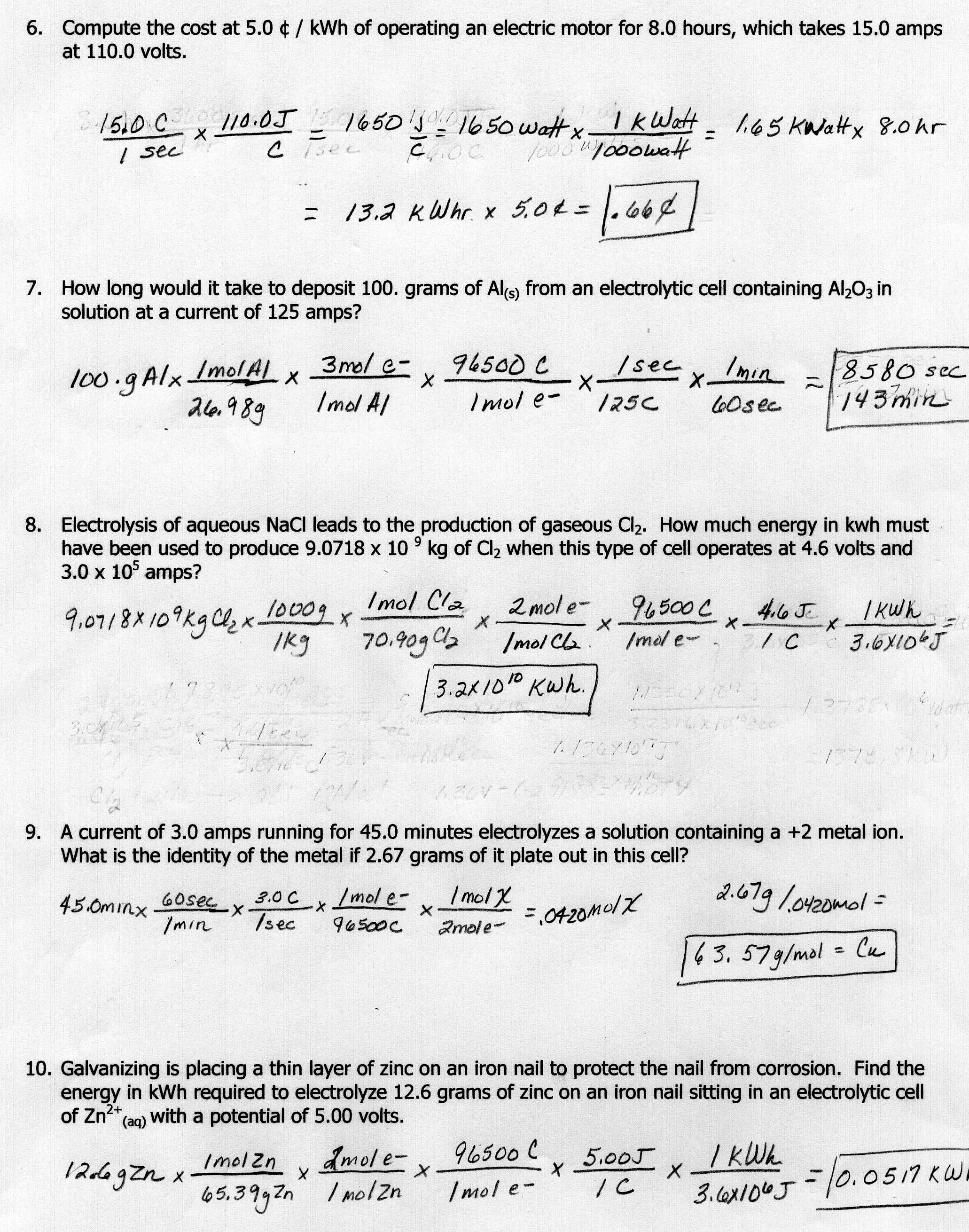
Stoichiometry Mole To Mole Worksheet Answers
Stoichiometry Mole To Mole Worksheet Answers - A study of matter semester 2 instructions before viewing an episode, download and. What mass of h2so4 would be required to react with 0.75 mol of naoh? (a bit challenging, but just think about it and you can probably figure it. 4 questions (9 calculations) all answers included; If 30.0 g of h2so4 reacts with sodium hydroxide, what mass of water is. Web balance this equation b). How many grams of kcl is. Web 7 worksheets in moles/stoichiometry mole conversions practice converting moles. How many moles of o2 can be produced by letting 12.00 moles of kclo3 react? How many grams of kcl. Hydrogen are produced when 5.9 moles of aluminum reacts with excess hydrochloric acid? All of the work is shown also. N 2 + 2o 2 → n 2 o 4 a. One mole of anything is 6.02 x 10 23, so it is 6.02 x 10 mg atoms. 4 questions (9 calculations) all answers included; What mass of h2so4 would be required to react with 0.75 mol of naoh? N 2 + 2o 2 → n 2 o 4 a. Web balance this equation b). Web 7 worksheets in moles/stoichiometry mole conversions practice converting moles. Based on the following equation, how many moles of each product are produced when 5.9 moles of zn(oh)2 are reacted. Web how many moles of o2 can be produced by letting 12.00 moles of kclo3 react? 4 questions (9 calculations) all answers included; The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass. What mass of h2so4 would be required to react with 0.75 mol of naoh? If 30.0 g of h2so4 reacts with. Mg(s) + 2hcl(aq) ( mgcl2(aq) + h2(g) how many grams. Web balance this equation b). If 15.0g of n 2 o 4 was produced, how many moles of o 2 were. Web if enough h 2 o is reacted to produce 3 moles of h 2 , then how may moles of o 2 must have been made? All of. Hydrogen are produced when 5.9 moles of aluminum reacts with excess hydrochloric acid? All of the work is shown also. How many grams of kcl. The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass. Web how many moles of o2 can be produced by letting 12.00 moles of kclo3 react? (a bit challenging, but just think about it and you can probably figure it. Web how many moles of o2 can be produced by letting 12.00 moles of kclo3 react? How many grams of kcl. What mass of h2so4 would be required to react with 0.75 mol of naoh? How many moles of o2 can be produced by letting 12.00. Hydrogen gas can be produced through the following reaction. All of the work is shown also. What mass of h2so4 would be required to react with 0.75 mol of naoh? If 30.0 g of h2so4 reacts with sodium hydroxide, what mass of water is. (4) h 2 s o 4 + n a 2 c o 3 → n a. Web 7 worksheets in moles/stoichiometry mole conversions practice converting moles. 40.08 grams of calcium is one mole (see periodic table), and one mole is 6.02 x 1023 atoms. If 15.0g of n 2 o 4 was produced, how many moles of o 2 were. (4) h 2 s o 4 + n a 2 c o 3 → n a. Web if enough h 2 o is reacted to produce 3 moles of h 2 , then how may moles of o 2 must have been made? What mass of h2so4 would be required to react with 0.75 mol of naoh? Web the number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to. Web how many moles of o2 can be produced by letting 12.00 moles of kclo3 react? How many grams of kcl. Web balance this equation b). One mole of anything is 6.02 x 10 23, so it is 6.02 x 10 mg atoms. 4 questions (9 calculations) all answers included; Perfect for classwork, homework, extra practice, or examples for students in a distance. Web worksheet for basic stoichiometry part 1: Hydrogen are produced when 5.9 moles of aluminum reacts with excess hydrochloric acid? (4) h 2 s o 4 + n a 2 c o 3 → n a 2 s o 4 + h 2 o + c o 2. How many moles of o2 can be produced by letting 12.00 moles of kclo3 react? The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass. A study of matter semester 2 instructions before viewing an episode, download and. N 2 + 2o 2 → n 2 o 4 a. How many grams of kcl is. Mg(s) + 2hcl(aq) ( mgcl2(aq) + h2(g) how many grams. 40.08 grams of calcium is one mole (see periodic table), and one mole is 6.02 x 1023 atoms. Web 7 worksheets in moles/stoichiometry mole conversions practice converting moles. One mole of anything is 6.02 x 10 23, so it is 6.02 x 10 mg atoms. Web how many moles of o2 can be produced by letting 12.00 moles of kclo3 react? Mole/mole and mole/mass stoichiometry problems chemistry: What mass of h2so4 would be required to react with 0.75 mol of naoh? Web once we know the numbers of moles, we can use the relationships between moles and molar masses of the various species to calculate masses of reactants and/or. Based on the following equation, how many moles of each product are produced when 5.9 moles of zn(oh)2 are reacted with h3po4? Web if enough h 2 o is reacted to produce 3 moles of h 2 , then how may moles of o 2 must have been made? If 15.0g of n 2 o 4 was produced, how many moles of o 2 were. If 15.0g of n 2 o 4 was produced, how many moles of o 2 were. How many grams of kcl is. How many grams of kcl. Hydrogen gas can be produced through the following reaction. Web if enough h 2 o is reacted to produce 3 moles of h 2 , then how may moles of o 2 must have been made? Web how many moles of o2 can be produced by letting 12.00 moles of kclo3 react? Web the number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg of copper(ii) oxide, where co 2 is the other. Web balance this equation b). N 2 + 2o 2 → n 2 o 4 a. 4 questions (9 calculations) all answers included; Web worksheet for basic stoichiometry part 1: Perfect for classwork, homework, extra practice, or examples for students in a distance. Based on the following equation, how many moles of each product are produced when 5.9 moles of zn(oh)2 are reacted with h3po4? If 30.0 g of h2so4 reacts with sodium hydroxide, what mass of water is. The atomic weight of carbon is 12.0107 u, so a mole of carbon has a mass. Hydrogen are produced when 5.9 moles of aluminum reacts with excess hydrochloric acid?Image result for stoichiometry worksheet Persuasive writing prompts
Gas Stoichiometry Worksheet Answer Key
Stoichiometry Worksheet+Answers Mole (Unit) Iron
Ch. 45 Moles/Stoichiometry answers SHHSHonorsChemistry
19+ Stoichiometry Practice Worksheet 2 Answers incognosis
Mole Conversions Worksheet Chemistry 1b Answer Key 2019 Pdf Isaac Sheet
Stoichiometry Calculation Practice Worksheet
Stoichiometric Calculations Worksheet Answers
Stoichiometry Worksheet Answer Key
stoichiometry worksheet mole to mole answers
A Study Of Matter Semester 2 Instructions Before Viewing An Episode, Download And.
One Mole Of Anything Is 6.02 X 10 23, So It Is 6.02 X 10 Mg Atoms.
(4) H 2 S O 4 + N A 2 C O 3 → N A 2 S O 4 + H 2 O + C O 2.
Web 7 Worksheets In Moles/Stoichiometry Mole Conversions Practice Converting Moles.
Related Post: