Stoichiometry Percent Yield Worksheet
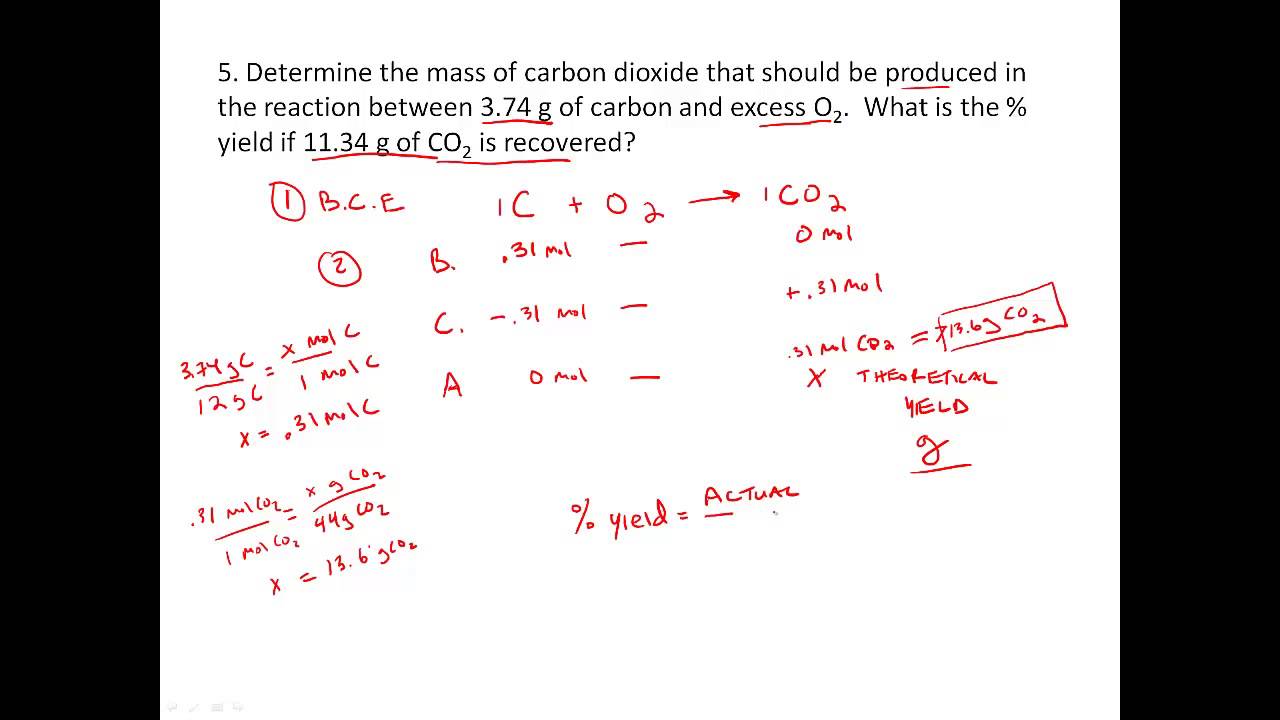
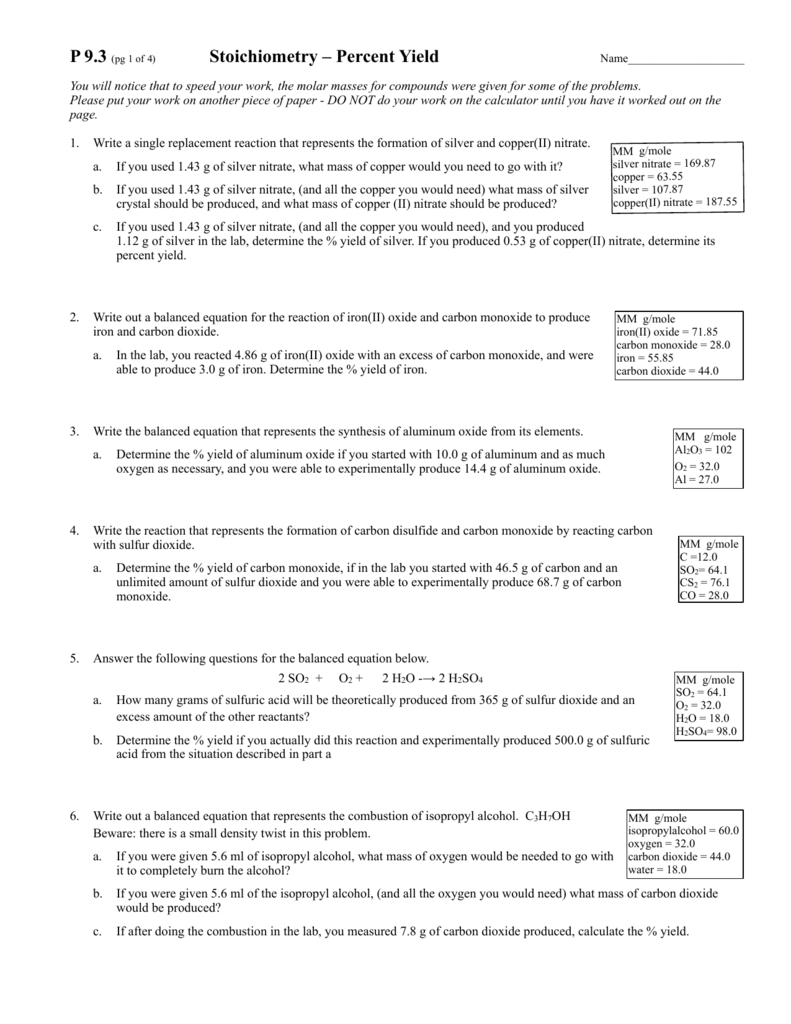
Stoichiometry Percent Yield Worksheet - Web according to the stoichiometry, the theoretical yield is 11.5 grams. Students will study the reaction of lead (ii) nitrate and potassium iodide. Web limiting reactant and percentage yield. Web percent yield “slaked lime,” ca(oh)2, is produced when water reacts with “quick lime,” cao. In this lesson, we will learn stoichiometry percent yield limiting reactants the following diagram shows the definition for percent yield. Web view stoichiometry worksheet 2 percent yield.pdf from chem 101 at gen douglas macarthur senior high school. 2060 g = actual yield cao + h2o ca(oh) 2 2400 g excess x g = theoretical yield mol cao Web another limiting reagent worksheet: Solution the provided information identifies copper sulfate as the limiting reactant, and so the theoretical yield is found by the approach illustrated in the previous module, as shown here: 1) 1 n 2 + 3 f 2 2 nf 3 2) 2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 3) 1 hbr + 1 khco 3 1 h 2 o + 1 kbr + 1 co 2 4) 2 gabr 3 + 3 na 2 so 3 1 ga 2 (so 3) 3 + 6 nabr 5) 3 sno + 2 nf 3 3 snf 2 + 1 n 2 o 3 using the following equation: Scroll down the page for more examples and solutions of how to find percent yield. In this lesson, we will learn stoichiometry percent yield limiting reactants the following diagram shows the definition for percent yield. Percent yield by chemistry corner this ngss aligned lesson builds on the stoichiometry and limiting and excess reactants concepts. If you start with 2 400. Web determine the theoretical and percent yield for this reaction? Web according to the stoichiometry, the theoretical yield is 11.5 grams. Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. 2060 g = actual yield cao + h2o ca(oh) 2 2400 g excess x g =. Web determine the theoretical and percent yield for this reaction? This resource includes a full answer key. Solution the provided information identifies copper sulfate as the limiting reactant, and so the theoretical yield is found by the approach illustrated in the previous module, as shown here: , as shown, if thewhat mass of f 2 is needed to produce 120.0. , as shown, if thewhat mass of f 2 is needed to produce 120.0 g of pf 3 reaction has a 78.1% yield? Web stoichiometry online worksheet for highschool. Multiplying this by 0.650, you get 7.48 grams. Web finding limiting reactant & The molar mass of the compound is 500 + 5 g/mole. This is part of a larger stoichiometry worksheet bundle that. Practice the calculations to find the limiting reagents and yields. Web another limiting reagent worksheet: This resource includes a full answer key. Fepo4 + na2so4 ( fe2(so4)3 + na3po4 35.25 g co2 and 14.65 g so2 are produced. Percent yield by chemistry corner this ngss aligned lesson builds on the stoichiometry and limiting and excess reactants concepts. % yield = actual yield x 100 theoretical yeild 1) balance the equation for the reaction of iron (iii) phosphate with sodium sulfate to make iron (iii) sulfate and sodium phosphate. If. By using stoichoimetry, we can predict the amount of product that will form in a chemical reaction, based on the amount of each reactant that we are starting with. The molar mass of the compound is 500 + 5 g/mole. Web view stoichiometry worksheet 2 percent yield.pdf from chem 101 at gen douglas macarthur senior high school. Web percent yield. When you divide actual yield by theoretical yield you get a decimal percentage known as the percent yield of a reaction. Web percent yield 2013 answers.notebook 2 february 28, 2013 = mass actual yield mass theoretical yield % yield x 100% the actual yield is often given in the problem.is occasionally referred to as the experimental yield. Web percent yield. You can do the exercises online or download the worksheet as pdf. This is part of a larger stoichiometry worksheet bundle that. Solution the provided information identifies copper sulfate as the limiting reactant, and so the theoretical yield is found by the approach illustrated in the previous module, as shown here: Web determine the theoretical and percent yield for this. Using theoretical and actual yields to determine whether the reaction was a success. If you start with 2 400 g of quick lime, add excess water, and produce 2 060 g of slaked lime, what is the percent yield of the reaction? The links to the corresponding topics are given below. Limiting reactants and percentage yield. Web according to the. The molar mass of the compound is 500 + 5 g/mole. Using theoretical and actual yields to determine whether the reaction was a success. Web percent yield 2013 answers.notebook 2 february 28, 2013 = mass actual yield mass theoretical yield % yield x 100% the actual yield is often given in the problem.is occasionally referred to as the experimental yield. Scroll down the page for more examples and solutions of how to find percent yield. Web view stoichiometry worksheet 2 percent yield.pdf from chem 101 at gen douglas macarthur senior high school. Web what is the percent yield? Web according to the stoichiometry, the theoretical yield is 11.5 grams. 28.50 g sample of a compound of carbon, sulfur, hydrogen, and oxygen is burned. Web calculate the percent yield of cl2. You can do the exercises online or download the worksheet as pdf. Web determine the theoretical and percent yield for this reaction? Practice the calculations to find the limiting reagents and yields. This resource includes a full answer key. When you divide actual yield by theoretical yield you get a decimal percentage known as the percent yield of a reaction. X 100 or the theoretical yeild 1) balance the equation for the reaction of iron (iii) phosphate with sodium sulfate to make iron (ii). Web stoichiometry online worksheet for highschool. Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. 2 h 3 po 4 + 3 mgco 3 mg 3 (po 4) 2 + 3 co 2 + 3 h 2 o ans: 35.25 g co2 and 14.65 g so2 are produced. 1.274 g cuso 4 × 1 mol cuso 4 159.62 g cuso 4 × 1 mol cu 1 mol cuso 4 × 63.55 g cu 1 mol cu = 0.5072 g cu You can do the exercises online or download the worksheet as pdf. Web what is the percent yield? If you start with 2 400 g of quick lime, add excess water, and produce 2 060 g of slaked lime, what is the percent yield of the reaction? The amount of product actually produced is called the actual yield. Part two of the limiting reagent saga. 1) 1 n 2 + 3 f 2 2 nf 3 2) 2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 3) 1 hbr + 1 khco 3 1 h 2 o + 1 kbr + 1 co 2 4) 2 gabr 3 + 3 na 2 so 3 1 ga 2 (so 3) 3 + 6 nabr 5) 3 sno + 2 nf 3 3 snf 2 + 1 n 2 o 3 using the following equation: Web percent yield 2013 answers.notebook 2 february 28, 2013 = mass actual yield mass theoretical yield % yield x 100% the actual yield is often given in the problem.is occasionally referred to as the experimental yield. 28.50 g sample of a compound of carbon, sulfur, hydrogen, and oxygen is burned. 35.25 g co2 and 14.65 g so2 are produced. This is part of a larger stoichiometry worksheet bundle that. 2 fepo4 + 3 na2so4 1 fe2(so4)3 + 2 na3po4 , as shown, if thewhat mass of f 2 is needed to produce 120.0 g of pf 3 reaction has a 78.1% yield? Web the calculated or expected amount of product is called the theoretical yield. Web stoichiometry online worksheet for highschool. Web determine the theoretical and percent yield for this reaction? Web according to the stoichiometry, the theoretical yield is 11.5 grams.Stoichiometry practice Percent Yield problems YouTube
14 Stoichiometry Worksheet 2 Answer Key /
Limiting Reagent Worksheet 1 Answers Percent Yield Worksheet
Stoichiometry Percent Yield
Stoichiometry Worksheet 2 Percent Yield
Stoichiometry Worksheet 2 Percent Yield worksheet
Stoichiometry Percent Yield Worksheet With Answers worksheet
Printables. Percent Yield Calculations Worksheet. Messygracebook
Calculate Percent Yield with Ideal Stoichiometry Practice 1 YouTube
Stoichiometry Worksheet 2 Percent Yield
This Predicted Amount Of Product Based On Stoichiometry Is Called Theoretical Yield.
This Is Called The Theoretical Yield , The Maximum Amount Of Product That Could Be Formed From The Given Amounts Of Reactants.
2 H 3 Po 4 + 3 Mgco 3 Mg 3 (Po 4) 2 + 3 Co 2 + 3 H 2 O Ans:
% Yield = Actual Yield X 100 Theoretical Yeild 1) Balance The Equation For The Reaction Of Iron (Iii) Phosphate With Sodium Sulfate To Make Iron (Iii) Sulfate And Sodium Phosphate.
Related Post: