Stoichiometry Practice 2 Worksheet Answers
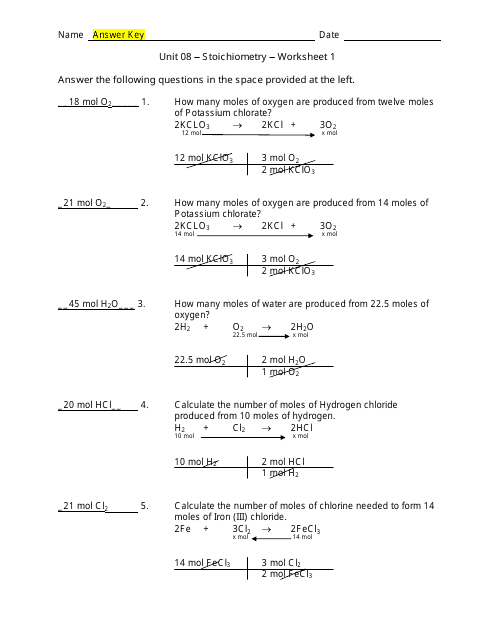
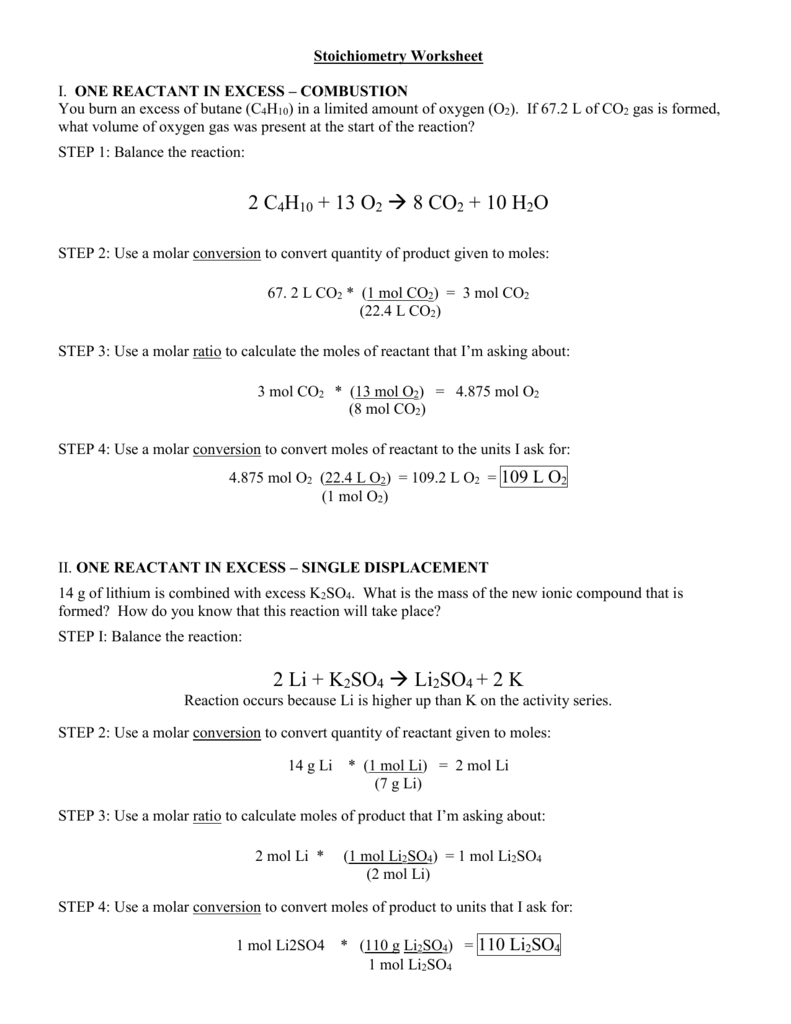
Stoichiometry Practice 2 Worksheet Answers - 3 kcl = mol kclo 3 = 0 mol fe = mol fe 2. Answer keys are included for all worksheets. Web stoichiometry worksheet 2 answer key 1. 15.0g n 2 o 4 1 mol n 2 o 4 2 mol o 2 92.0g n 2 o 4 1 mol n 2 o 4 = 0.326 mol o 2 b. Web it is prepared by the reaction of pure sand, sio 2, with carbon at high temperature. If it takes 27.4 ml of 0.768 m n a o h to titrate 16.7 ml of h 2 s o 4, what is the concentration of the h 2 s o 4 solution? How many moles of o2 can be produced by letting 12.00 moles of kclo3 react? B) how many molecules of iron (iii) oxide can be produced from 13.5 moles fe? More fun for the whole chemist family. Web stoichiometry practice worksheet: A) how many moles of iron would be needed to react with 3.82 moles of oxygen? Mass to mass worksheet 2.1 ”. Web stoichiometry worksheets and online activities. The topics for each worksheet is as follows: If 15.0g of n 2 o 4 was produced, how many moles of o 2 were required? The topics for each worksheet is as follows: If it takes 27.4 ml of 0.768 m n a o h to titrate 16.7 ml of h 2 s o 4, what is the concentration of the h 2 s o 4 solution? H2so4 + 2naoh ( na2so4 + 2h2o. Calculate the mass of nh3 that can be produced from the. N 2 + 2o 2 → n 2 o 4 a. How many moles of water are produced when 57 moles of nitrogen are made? (a bit challenging, but just think about it and you can probably figure it out) stoichiometry worksheet and key. The overall reaction in the body is c6h12o6+ 6o2 6co2 + 6h2o calculate the number of. Web if enough h 2 o is reacted to produce 3 moles of h 2 , then how may moles of o 2 must have been made? The overall reaction in the body is c6h12o6+ 6o2 6co2 + 6h2o calculate the number of moles of oxygen needed to oxidize 12.5 g of c6h12o6. Balance the equation first) q2. Balancing equations. A) how many moles of iron would be needed to react with 3.82 moles of oxygen? N 2 + 2o 2 → n 2 o 4 a. 2 / 10 (or 1 / 5) 2. X = 18.00 mol of o 2 3. Web if enough h 2 o is reacted to produce 3 moles of h 2 , then. Calculate the number of moles of naoh that are needed to react with 500 g of h 2 so 4 according to the following equation: Carbon monoxide, co, is the other product of this reaction. H 2 s o 4 + n a o h → n a 2 s o 4 + h 2 o. 7) using the following. If 15.0g of n 2 o 4 was produced, how many moles of o 2 were required? Using molarity and stoichiometry together. Balancing equations and simple stoichiometry: Calculate the mass of nh3 that can be produced from the reaction of 125 g of ncl3 according to the following equation: How many grams of kcl is produced from 2.50 g of. This is a bundle of homework worksheets that i use with my classes when i teach stoichiometry. N 2 + 2o 2 → n 2 o 4 a. The kclo 3 / o 2 molar ratio is 2/3. How many moles of water are produced when 57 moles of nitrogen are made? Balance the equation first) q2. 3 kcl = mol kclo 3 = 0 mol fe = mol fe 2. ___ fe + ___ o2 ( ___ fe2o3. Mol o 2 = mol o 2. How many grams of kcl is produced from 1.00 g of cl2 and excess k ? Calculate the mass of nh3 that can be produced from the reaction of 125 g. Ncl 3 + 3 h 2 o nh 3 + 3 hocl. 2h2 + o2 ( 2h2o. H2so4 + 2naoh ( na2so4 + 2h2o. 3 n2(g) + 4 h2o(g). C) how many moles of o2 are needed to produce 34.7 g of fe2o3? Calculate the number of moles of naoh that are needed to react with 500 g of h 2 so 4 according to the following equation: 3 kcl = mol kclo 3 = 0 mol fe = mol fe 2. 15.0g n 2 o 4 1 mol n 2 o 4 2 mol o 2 92.0g n 2 o 4 1 mol n 2 o 4 = 0.326 mol o 2 b. Web it is prepared by the reaction of pure sand, sio 2, with carbon at high temperature. 500.0 g c 6 h 12 o 6 ¸¸ ¹ · ¨¨ © § ¸¸ ¹. 7) using the following equation: Answer keys are included for all worksheets. What mass of h2o is formed when h2 reacts with 384 g of o2? If it takes 27.4 ml of 0.768 m n a o h to titrate 16.7 ml of h 2 s o 4, what is the concentration of the h 2 s o 4 solution? Web stoichiometry worksheet 2 answer key 1. H2so4 + 2naoh ( na2so4 + 2h2o. X = 18.00 mol of o 2 3. Calculate the number of moles of naoh that are needed to react with 500.0 g of h2so4 according to the following equation: N a o h + h c l → h 2 o + n a c l. Web chm 130 stoichiometry worksheet key 1. Each worksheet is clearly labeled for each lesson and is fully adaptable to any chemistry classroom. Using molarity and stoichiometry together. ___ fe + ___ o2 ( ___ fe2o3. Pb(so4)2 + 4 lino3 pb(no3)4 + 2 li2so4. Balance the equation first) q2. 2) ammonia is synthesized from hydrogen and nitrogen according to the. Web stoichiometry worksheet 2 answer key 1. H 2 so 4 + 2 naoh na 2 so 4 + 2 h 2 o. 7) using the following equation: O 2 = 12.00 mol kclo 3 / x = 18.00 mol. C 6 h 12 o 6 (s) 2 c 2 h 5 oh (l) + 2 co 2 (g) a. A) how many moles of iron would be needed to react with 3.82 moles of oxygen? All of the work is shown also. If it takes 27.4 ml of 0.768 m n a o h to titrate 16.7 ml of h 2 s o 4, what is the concentration of the h 2 s o 4 solution? ___ fe + ___ o2 ( ___ fe2o3. How many moles of h2 would be required to produce 5.0 moles of water? How many grams of lithium nitrate will be needed to make 250 grams of lithium sulfate, assuming that you have an adequate amount of lead (iv) sulfate to do the reaction? Web if enough h 2 o is reacted to produce 3 moles of h 2 , then how may moles of o 2 must have been made? How many moles of o2 can be produced by letting 12.00 moles of kclo3 react? Just what it sounds like. 2 / 8 (or 1 / 4) e.14 Stoichiometry Worksheet 2 Answer Key /
Intro To Stoichiometry Worksheet
Worksheet For Basic Stoichiometry Answers
Printables. Stoichiometry Worksheets. Tempojs Thousands of Printable
Gas Stoichiometry Worksheet Doc Worksheets Joy
volume to volume stoichiometry worksheet
Stoichiometry Worksheet 2 Answers
19+ Stoichiometry Practice Worksheet 2 Answers incognosis
Worksheet On Stoichiometry With Answers Herbalied
Ch. 45 Moles/Stoichiometry answers SHHSHonorsChemistry
Calculate The Number Of Moles Of Naoh That Are Needed To React With 500 G Of H 2 So 4 According To The Following Equation:
The Topics For Each Worksheet Is As Follows:
Web It Is Prepared By The Reaction Of Pure Sand, Sio 2, With Carbon At High Temperature.
Pb(So4)2 + 4 Lino3 Pb(No3)4 + 2 Li2So4.
Related Post: