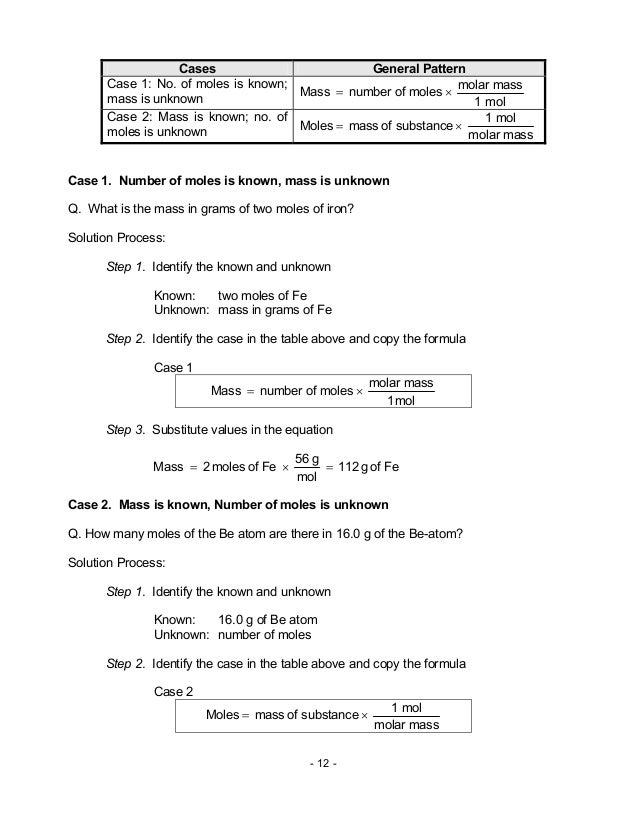
Stoichiometry Problems Chem Worksheet 12-2 Answers
Stoichiometry Problems Chem Worksheet 12-2 Answers - ___ cacl2 + ___ agno3. A balanced equation and the amount of the starting materials. July 30, 2019 by chess93. Calculate the grams of zinc chloride produced if 0.238 grams of zinc react completely. Web consider the reaction of zinc metal with hydrochloric acid, hcl(aq). Silver nitrate reacts with barium chloride to form silver chloride and barium nitrate. Web san juan unified school district / homepage Web if step i gives n 2 o 2 in adequate amount, steps 1 and 2 combine to give 2 no + h 2 h 2 o + n 2 o. How many liters of oxygen at stp are required for the combustion of 1.4 g of magnesium? Combine steps 1 and 2 with step 3, which occurs by supposition in a rapid fashion, to give the appropriate stoichiometry. Write the balanced equation and determine the information requested. If 39.02 grams of barium chloride are reacted in an excess of silver nitrate, how many representative particles (and what type. If 25 g of albr. The links to the corresponding topics are. The number of moles and the mass (in grams) of chlorine, cl 2, required to react with 10.0. If 39.02 grams of barium chloride are reacted in an excess of silver nitrate, how many representative particles (and what type. Csbr + al(oh) 3 albr 3 + csoh a. How many molecules of oxygen are required to react with 174 g of carbon monoxide? Web 50 stoichiometry problems worksheet answers. Answer keys are included for all worksheets. Calculate the grams of zinc chloride produced if 0.238 grams of zinc react completely. Show your work, including proper units, to earn full credit. __ mg + __ o2 __ mgo. Web san juan unified school district / homepage Calculate the moles of hcl needed to react completely with 8.25 moles of zinc. Don't worry about state symbols in these reactions. __ mg + __ o2 __ mgo. N a o h + h c l → h 2 o + n a c l. Web figure 12.2.1 an expanded flowchart for stoichiometric calculations either the masses or the volumes of solutions of reactants and products can be used to determine the amounts. N a o h + h c l → h 2 o + n a c l. Sample stoichiometry worksheet 9 examples in word pdf from stoichiometry problems worksheet answers , image source: Calculate the moles of hcl needed to react completely with 8.25 moles of zinc. If it takes 27.4 ml of 0.768 m n a o h to. Web 50 stoichiometry problems worksheet answers. The moisture will be weighed as part of the product, giving a falsely elevated actual yield. Show your work, including proper units, to earn full credit. This reaction corresponds to the observed rate law. Combine steps 1 and 2 with step 3, which occurs by supposition in a rapid fashion, to give the appropriate. If 39.02 grams of barium chloride are reacted in an excess of silver nitrate, how many representative particles (and what type. The links to the corresponding topics are. Web consider the reaction of zinc metal with hydrochloric acid, hcl(aq). How many liters of oxygen at stp are required for the combustion of 1.4 g of magnesium? Calculate the percent yield. + 4 lin03 +2 li2s04 July 30, 2019 by chess93. The topics for each worksheet is as follows: Web complete stoichiometry problems chem worksheet 12 2 online with us legal forms. Web 50 stoichiometry problems worksheet answers. This is a bundle of homework worksheets that i use with my classes when i teach stoichiometry. Balance the equation first) q2. Write the equation for this reaction, then balance the equation. Answer keys are included for all worksheets. Calculate the grams of zinc chloride produced if 0.238 grams of zinc react completely. Show your work, including proper units, to earn full credit. Calculate the moles of hcl needed to react completely with 8.25 moles of zinc. Write the equation for this reaction, then balance the equation. How many molecules of oxygen are required to react with 174 g of carbon monoxide? ___ cacl2 + ___ agno3. The moisture will be weighed as part of the product, giving a falsely elevated actual yield. Write the balanced equation and determine the information requested. Web stoichiometry practice problems **balance the following equations first, then answer the questions: A) k 2 so4 (s) + bacl2 (aq) baso4 (s) + kcl (aq) This is a bundle of homework worksheets that i use with my classes when i teach stoichiometry. Solve each of the following problems. Arithmetic worksheet answers to equation worksheet answers for the research section of the biology math. Great for extra practice worksheets! How many grams of silver chloride are produced when 45 g of calcium chloride react with excess silver nitrate? The links to the corresponding topics are. Choose the mole ratio to convert the number of moles of al2o3 into moles of al update this answer! 11 answer 7.4 mol al work step by step list the six possible mole ratios of the chemical equation. Csbr + al(oh) 3 albr 3 + csoh a. ___ ca(no3)2 + ___ agcl. 2 agno3 + bacl2 ! Calculate the grams of zinc chloride produced if 0.238 grams of zinc react completely. Write the equation for this reaction, then balance the equation. The topics for each worksheet is as follows: 2 no + h 2 h 2 o + n 2 o. Assuming 1l of total solution, calculate the concentration of each ion left in solution after reaction. 2 agcl + ba (no3)2 b. N a o h + h c l → h 2 o + n a c l. This is a bundle of homework worksheets that i use with my classes when i teach stoichiometry. Solve each of the following problems. Sample stoichiometry worksheet 9 examples in word pdf from stoichiometry problems worksheet answers , image source: Web honors chemistry extra stoichiometry problems 1. How many moles of \ce {zncl2} znclx 2 will be produced from 23.0 \text { g} 23.0 g of \ce {zn} zn, assuming \ce {cucl2} cuclx 2 is available in excess? This reaction corresponds to the observed rate law. Arithmetic worksheet answers to equation worksheet answers for the research section of the biology math. Csbr + al(oh) 3 albr 3 + csoh a. Combine steps 1 and 2 with step 3, which occurs by supposition in a rapid fashion, to give the appropriate stoichiometry. The number of moles and the mass (in grams) of chlorine, cl 2, required to react with 10.0 g of sodium metal, na, to produce sodium chloride, nacl. 11 answer 7.4 mol al work step by step list the six possible mole ratios of the chemical equation. Web if step i gives n 2 o 2 in adequate amount, steps 1 and 2 combine to give 2 no + h 2 h 2 o + n 2 o. Calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of 1.45 g. Write the equation for this reaction, then balance the equation.Stoichiometry Problems Chem Worksheet 12 2 Answer Key
chemistry stoichiometry worksheet 2 answers
Stoichiometry Problems Chem Worksheet 12 2 Answer Key
Chm 130 Stoichiometry Worksheets
43 stoichiometry problems chem worksheet 12 2 answer key Worksheet
Stoichiometry Worksheet 2 Answers richinspire
Stoichiometry Problems Chem Worksheet 12 2 Answer Key
43 stoichiometry problems chem worksheet 12 2 answer key Worksheet
Stoichiometry Worksheet And Key Answers paladininspire
Stoichiometry Problems Chem Worksheet 12 2 Answers / Foothill High
Calculate The Grams Of Zinc Chloride Produced If 0.238 Grams Of Zinc React Completely.
If It Takes 27.4 Ml Of 0.768 M N A O H To Titrate 16.7 Ml Of H 2 S O 4, What Is The Concentration Of The H 2 S O 4 Solution?
The Percent Yield Will Also Be Lower.
The Moisture Will Be Weighed As Part Of The Product, Giving A Falsely Elevated Actual Yield.
Related Post: