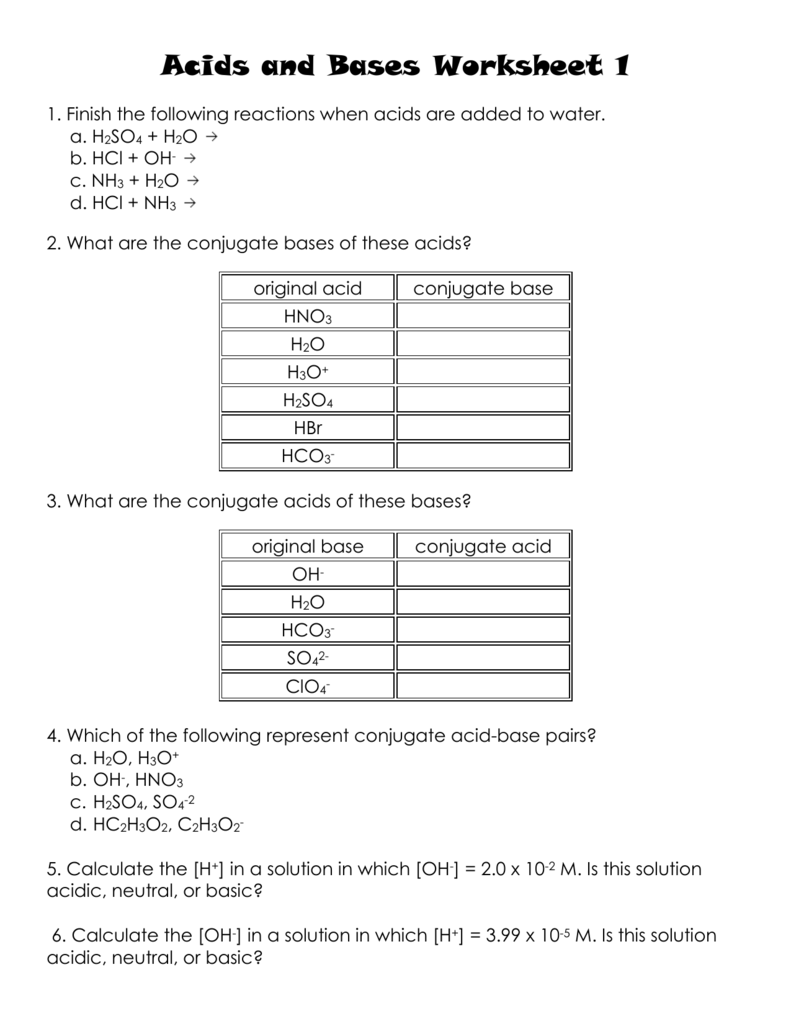
Strength Of Acids And Bases Worksheet Answers
Strength Of Acids And Bases Worksheet Answers - Click the card to flip 👆. A base that only partly ionizes in a solution is a _ weak _ base. Web acids and bases worksheet + answers. Web acids and bases 2 (worksheet) work in groups on these problems. Examples of acids are lemon juice and vinegar. Strong bases are good electrolytes because they completely ionize in water. Which of the following statements is correct? A bitter taste and a slippery feel are clues that a solution is probably a(n) _ base _. Are the anions (conjugate bases) of strong acids: Any base that dissociates completely in solution. Click the card to flip 👆. Web the rules for deciding if a base is strong or weak! Rank the bases by increasing base strength: You should try to answer the questions without referring to your textbook. Which of the following statements is correct? 4b) no equilibrium since the good acid and very strong base on the left will second the reaction completely to the right (i.e. Using the table of relative strengths of acids and bases to answer the following questions: Describe the difference between strong and weak acids and bases. Click the card to flip 👆. Which compounds from the list are. Which compounds from the list are n? Any acid that only partly dissociates in solution. Web assess the relative strengths of acids and bases according to their ionization constants; Which compounds from the list are basic? 4b) no equilibrium since the good acid and very strong base on the left will second the reaction completely to the right (i.e. Improving upon the capabilities of indicator paper, ph meters are able to quantitatively measure the acidity of a solution. Any base that dissociates completely in solution. Ha(aq) + h 2o(l) ⇌ h 3o + (aq) + a − (aq) All other bases are weak. Web _ indicators _ change color in the presence of an acid or a base. Which of the following is one of the strongest acids? Rank the bases by increasing base strength: Are the anions (conjugate bases) of weak acids: All ph values that follow are measurements of 0.010 m solutions. In some cases, you likewise realize All ph values that follow are measurements of 0.010 m solutions. Examples of a base substance are soap and baking soda. Web reading strategy (page 246) comparing and contrastingas you read, complete the diagram bycomparing and contrasting acids and bases. Assume the solutions are 0.1 m at room temperature. We have seen that the calculation of [h 3 o +]. Web the rules for deciding if a base is strong or weak! Understand the principles of buffer solutions. Rank the bases by increasing base strength: Which of the following statements is correct? Any acid that only partly dissociates in solution. Strengths of acids and bases. Which compounds from the list are acidic? Examples of acids are lemon juice and vinegar. The ph scale (page 247) Using the table of relative strengths of acids and bases to answer the following questions: Web the rules for deciding if a base is strong or weak! All ph values that follow are measurements of 0.010 m solutions. Which compounds from the list are n? Click the card to flip 👆. Web assess the relative strengths of acids and bases according to their ionization constants; A measure of the concentration of hydronium ions in a solution using a scale ranging from 0 to 14, with 0 being the most acidic and 14 being the. Web acids and bases 2 (worksheet) work in groups on these problems. Click the card to flip 👆. Using the table of relative strengths of acids and bases to answer the. Web acids and bases 2 (worksheet) work in groups on these problems. Define the ph scale and use it to describe acids and bases. Which compounds from the list are acidic? We have seen that the calculation of [h 3 o +] and ph for solutions of strong acids and base. Are the anions (conjugate bases) of strong acids: Examples of a base substance are soap and baking soda. Examples of acids are lemon juice and vinegar. _ strength _ of a solution refers to the ease with which an acid or base forms ions in solution. Web assess the relative strengths of acids and bases according to their ionization constants; Any base that dissociates completely in solution. 4.7 (37 reviews) get a hint. Click the card to flip 👆. In some cases, you likewise realize Web reading strategy (page 246) comparing and contrastingas you read, complete the diagram bycomparing and contrasting acids and bases. A measure of the concentration of hydronium ions in a solution using a scale ranging from 0 to 14, with 0 being the most acidic and 14 being the. Acids and bases do not all demonstrate the same degree of chemical activity in solution. Web 4a) k > 1 since the stronger acid and base on the left will favour the reaction to the right. You might not require more period to spend to go to the book commencement as well as search for them. Web know the relationships between structure types and acid strength. The reaction of an acid with water is given by the general expression: Web acids and bases worksheet + answers. Given the same initial concentration of each acid, the highest percent of ionization is acid a because it is the strongest acid. A base that only partly ionizes in a solution is a _ weak _ base. 4c) k ~ 1 since acids and bases on each side are of similar strengths. You might not require more period to spend to go to the book commencement as well as search for them. All ph values that follow are measurements of 0.010 m solutions. Web _ indicators _ change color in the presence of an acid or a base. Web strength of acids and bases worksheet answers this is likewise one of the factors by obtaining the soft documents of this strength of acids and bases worksheet answers by online. H2o, hno3, hocl, nh4 +. 4b) no equilibrium since the good acid and very strong base on the left will second the reaction completely to the right (i.e. A bitter taste and a slippery feel are clues that a solution is probably a(n) _ base _. Web acids, bases, and conjugates, miscellaneous. Web know the relationships between structure types and acid strength. Web assess the relative strengths of acids and bases according to their ionization constants; A solution of a weak acid or weak base. Web 4a) k > 1 since the stronger acid and base on the left will favour the reaction to the right.worksheet. Conjugate Acid Base Pairs Worksheet. Grass Fedjp Worksheet
Acid And Base Worksheet
Acids and Bases Worksheet Answers
Acids and bases worksheet 2 worksheet
Strength Of Acids Pogil Answers
Acid and Base Strength Worksheet
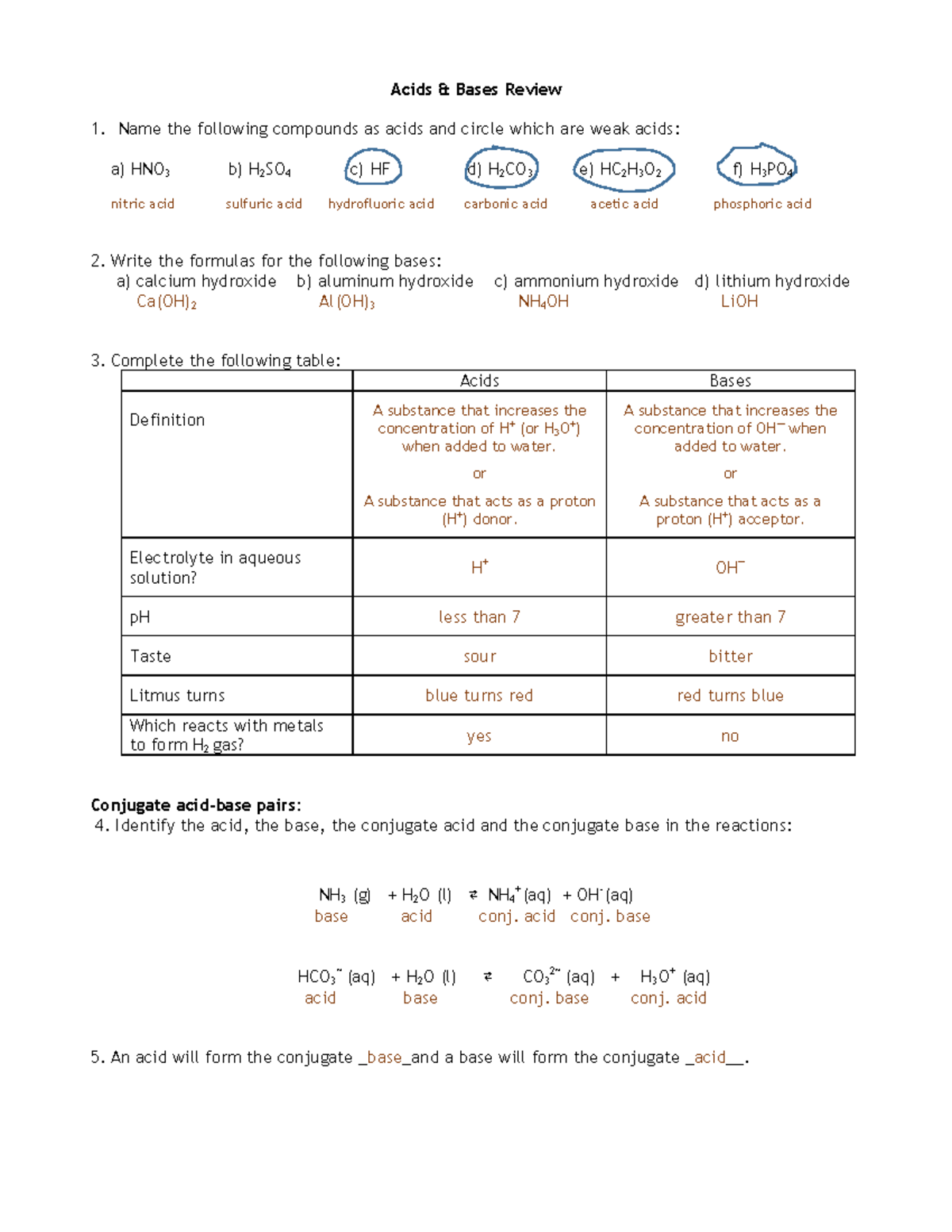
Acids and Bases Review Worksheet Answers Acids & Bases Review 1. Name
Acids And Bases In Solution Worksheet Answers Escolagersonalvesgui
Acid Base Worksheet 1
Acid And Base Worksheet Answers
4.7 (37 Reviews) Get A Hint.
Click The Card To Flip 👆.
By Reversing The Reaction In Which A Substance Acts As A Proton Donor, We See That The Product Is Itself A Proton Acceptor.
Are The Anions (Conjugate Bases) Of Strong Acids:
Related Post: