Synthesis And Decomposition Reactions Worksheet
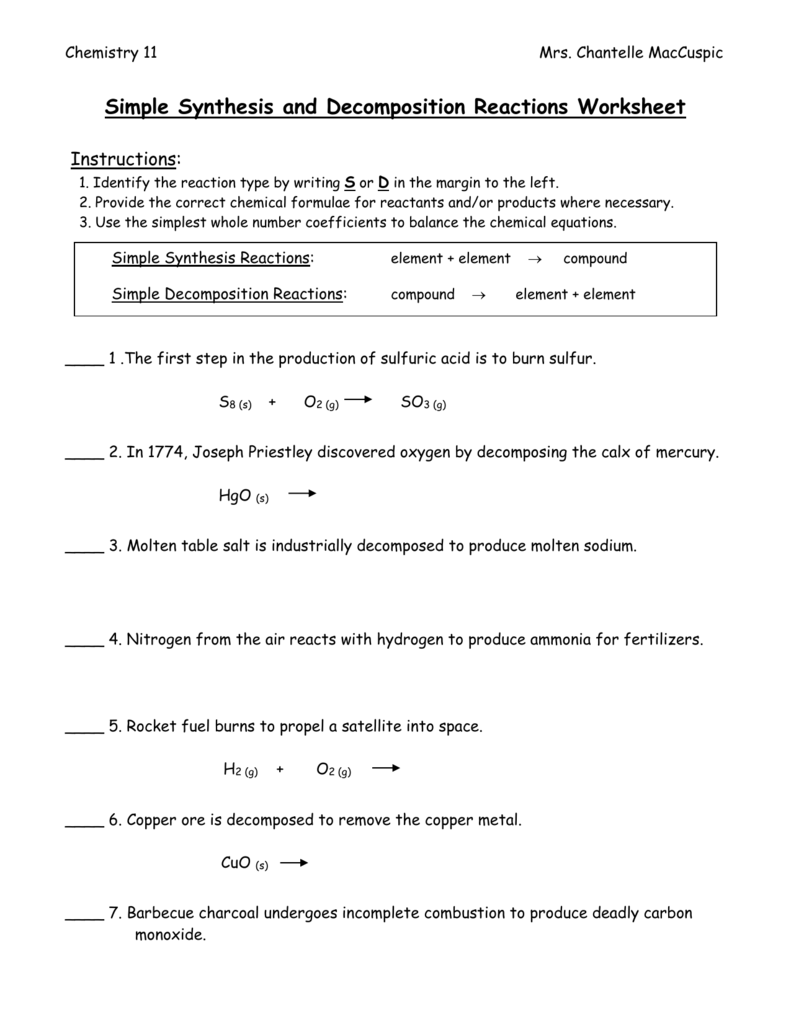
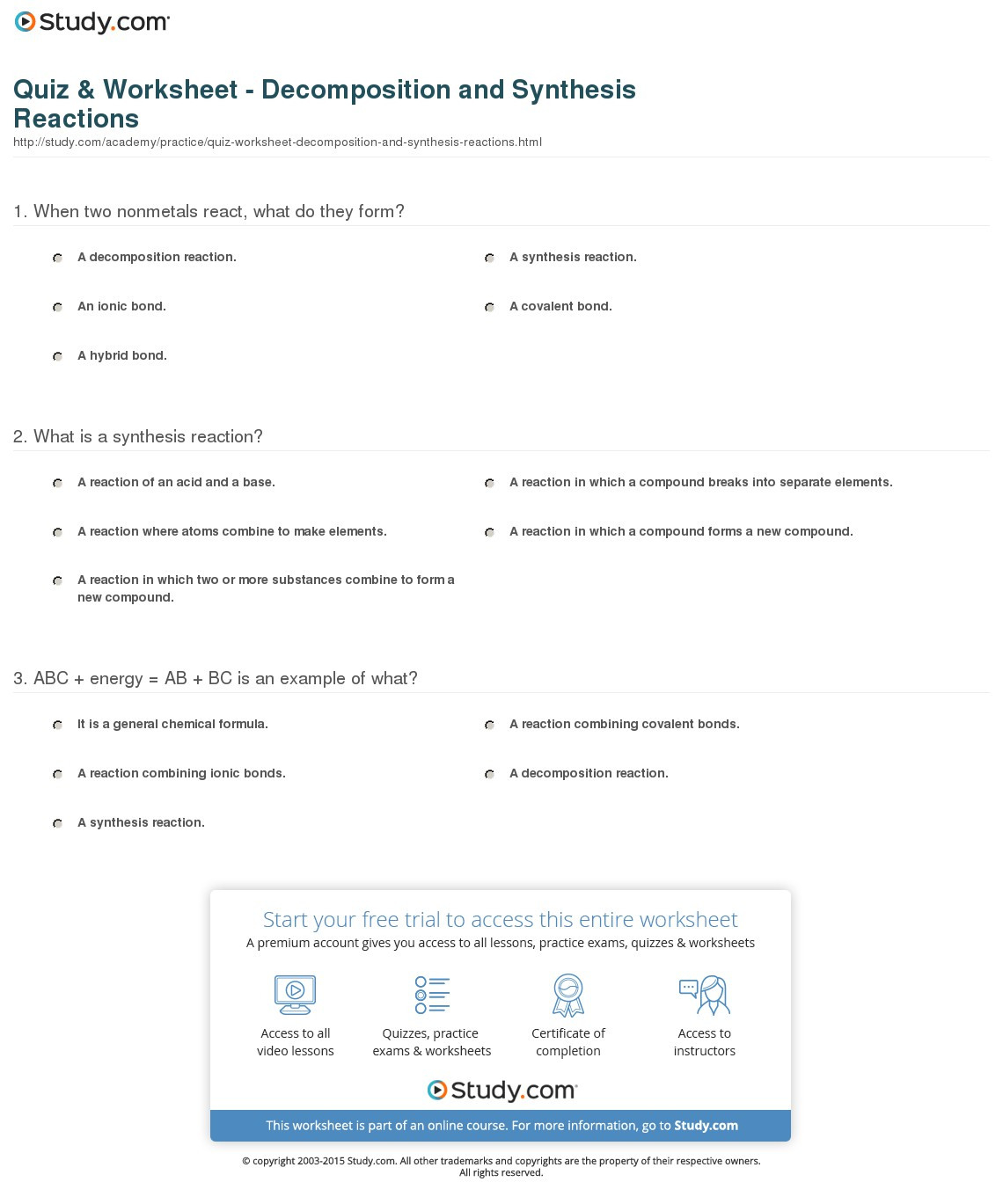
Synthesis And Decomposition Reactions Worksheet - Elements combine to form one compound or compounds combine to form one large compound. Phosphorous + oxygen à tetraphosphorous decaoxide. Note that the product is a gas. Web synthesis reactions are chemical reactions in which two or more substances react to form a new product. A + b à ab. Web up to 24% cash back synthesis and decomposition reactions 1. H 2 (g) + cl 2 (g)2 hcl. For each of thechemical reactions are listed below, completethe following: Web 1) identify each reaction as synthesis or decomposition and 2) write a balanced chemical equation for each reaction. Web synthesis and decomposition reactions. H 2 (g) + cl 2 (g)2 hcl. Web the general form of a synthesis reaction is written as: Web 1) identify each reaction as synthesis or decomposition and 2) write a balanced chemical equation for each reaction. For each of the following word equations, write a balanced chemical equation. A + b ab decomposition reactions are chemical reactions in. A + b ab decomposition reactions are chemical reactions in which a reactant breaks down into two ormore. A + b decomposition reactions are chemical reactions in which a reactant breaks down into two or more products. Worksheets are work 3 decomposition reactions, synthesis and. Web a worksheet that could be completed as individual work, small group student work or. Web this worksheet set includes 50+ problems for students to complete, including.identifying all five types of reactions :1) synthesis 2) decomposition3) single displacement4). Web write a balanced chemical equation for the synthesis reaction between hydrogen gas and chlorine gas, shown in figure 5. For each of the following word equations, write a balanced chemical equation. Web synthesis and decomposition reactions. Web up to 24% cash back synthesis and decomposition reactions 1. ____ nabr + ____ h3po4 ____ na3po4 + ____ hbr type of reaction: A + b decomposition reactions are chemical reactions in which a reactant breaks down into two or more products. Elements combine to form one compound or compounds combine to form one large compound. Web a synthesis. Web 1) identify each reaction as synthesis or decomposition and 2) write a balanced chemical equation for each reaction. Web a worksheet that could be completed as individual work, small group student work or teacher lead instruction that requires students to identify two types of chemical reactions. Phosphorous + oxygen à tetraphosphorous decaoxide. Web up to 24% cash back synthesis. Web up to 24% cash back synthesis and decomposition reactions 1. Web synthesis and decomposition reactions interactive and downloadable worksheets. It involves the combination of smaller atoms and /ormolecules into. ____ nabr + ____ h3po4 ____ na3po4 + ____ hbr type of reaction: H 2 (g) + cl 2 (g)2 hcl. Note that the product is a gas. Web synthesis reactions are chemical reactions in which two or more substances react to form a new product. Web the general form of a synthesis reaction is written as: ____ nabr + ____ h3po4 ____ na3po4 + ____ hbr type of reaction: For each of the following word equations, write a balanced chemical. Web the general form of a synthesis reaction is written as: Web synthesis and decomposition reactions interactive and downloadable worksheets. It involves the combination of smaller atoms and /ormolecules into. Web write a balanced chemical equation for the synthesis reaction between hydrogen gas and chlorine gas, shown in figure 5. Web balance the following equations and indicate the type of. H 2 (g) + cl 2 (g)2 hcl. A + b decomposition reactions are chemical reactions in which a reactant breaks down into two or more products. Synthesis, decomposition & combustion synthesis: Synthesis reactions release energy in the form of heat and light, so they. Note that the product is a gas. Web the general form of a synthesis reaction is written as: H 2 (g) + cl 2 (g)2 hcl. Note that the product is a gas. ____ nabr + ____ h3po4 ____ na3po4 + ____ hbr type of reaction: Worksheets are work 3 decomposition reactions, synthesis and. Note that the product is a gas. ____ nabr + ____ h3po4 ____ na3po4 + ____ hbr type of reaction: Phosphorous + oxygen à tetraphosphorous decaoxide. Web the general form of a synthesis reaction is written as: Web a synthesis reaction is a type of reaction in which multiple reactants combine to form a single product. Web the general form of a synthesis reaction is written as: Web a worksheet that could be completed as individual work, small group student work or teacher lead instruction that requires students to identify two types of chemical reactions. Hydrates ( slide notes ) * worksheet #1: Worksheets are work 3 decomposition reactions, synthesis and. 1) synthesis ( slide notes) *worksheet #2:. For each of the following word equations, write a balanced chemical equation. It involves the combination of smaller atoms and /ormolecules into. Web 1) identify each reaction as synthesis or decomposition and 2) write a balanced chemical equation for each reaction. For each of thechemical reactions are listed below, completethe following: The general form of a synthesis reaction is written as: Elements combine to form one compound or compounds combine to form one large compound. Web synthesis and decomposition reactions interactive and downloadable worksheets. Types of chemical reactions (* summary handout ): Synthesis reactions release energy in the form of heat and light, so they. A + b decomposition reactions are chemical reactions in which a reactant breaks down into two or more products. A + b à ab. Types of chemical reactions (* summary handout ): Web a worksheet that could be completed as individual work, small group student work or teacher lead instruction that requires students to identify two types of chemical reactions. 1) synthesis ( slide notes) *worksheet #2:. Web a synthesis reaction is a type of reaction in which multiple reactants combine to form a single product. Web up to 24% cash back synthesis and decomposition reactions 1. Synthesis, decomposition & combustion synthesis: Worksheets are work 3 decomposition reactions, synthesis and. Web the general form of a synthesis reaction is written as: Hydrates ( slide notes ) * worksheet #1: Web balance the following equations and indicate the type of reaction taking place: Elements combine to form one compound or compounds combine to form one large compound. Note that the product is a gas. Web this worksheet set includes 50+ problems for students to complete, including.identifying all five types of reactions :1) synthesis 2) decomposition3) single displacement4). Synthesis reactions release energy in the form of heat and light, so they. A + b decomposition reactions are chemical reactions in which a reactant breaks down into two or more products.Synthesis and Reactions WKS
Synthesis Reaction Worksheet —
And Synthesis Reactions Worksheet kamberlawgroup
Quiz Worksheet And Synthesis Reactions Study —
25 Fresh Synthesis And Reactions Worksheet Answers
9 Best Images of Bill Nye Chemical Reactions Worksheet Bill Nye
synthesis and reactions worksheet
Worksheet 1 Synthesis Reactions —
Neutralization Reactions Worksheets
5 Types Of Chemical Reactions Lab With Worksheet Answers
Magnesium + Oxygen Gas Magnesium.
For Each Of Thechemical Reactions Are Listed Below, Completethe Following:
A + B Ab Decomposition Reactions Are Chemical Reactions In Which A Reactant Breaks Down Into Two Ormore.
Web Synthesis And Decomposition Reactions.
Related Post: