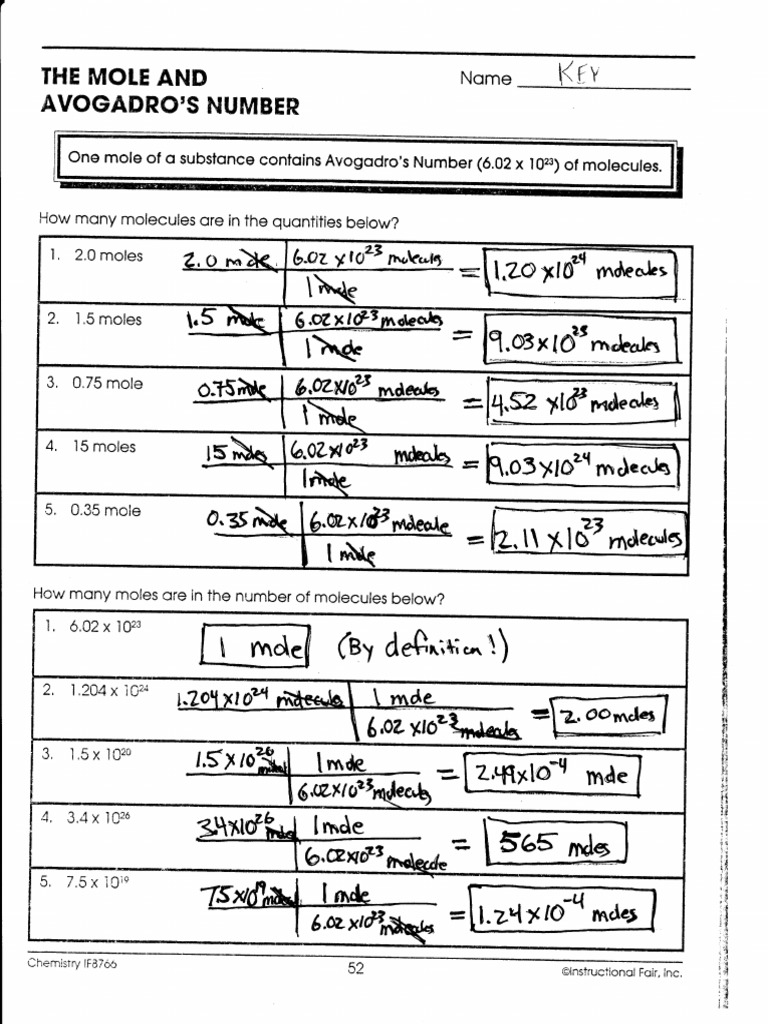
The Mole And Avogadro's Number Worksheet Answers
The Mole And Avogadro's Number Worksheet Answers - Just as a dozen apples is 12 apples, a mole of apples is 6.022 x 1023 apples. However, the only practical use for na is to have a more. What number of iron atoms, each weighing 55.847 u, is necessary to get 55.847 g of fe? Web this senior chemistry lesson package discusses the mole, avogadro’s number, molar mass and provides a lot of practice with the formulas to determine n and the number of. Web using avogadro’s number to convert from molecules or atoms to moles. Make sure to learn these vocabulary terms and the kinds of chemical formulas they indicate. Using the information in the table, calculate the number of moles in a \pu {2.03 kg} 2.03 kg sample of citric acid ( \ce {c6h8o7} cx 6hx 8ox 7). Web view the mole and avogadros number worksheet key.pdf from bchs 4325 at university of houston. K, ag, s) , an ion (e.g. Questions from the powerpoint lecture: Web a mole of objects contains avogadro's number, 6.022 x 1023, objects. Apples, stars in the sky, burritos. Just as a dozen apples is 12 apples, a mole of apples is 6.022 x 1023 apples. Web the relationships between formula mass, the mole, and avogadro’s number can be applied to compute various quantities that describe the composition of substances and.. Molar mass cabr2 = 199.886 g/mol 4. Web the relationships between formula mass, the mole, and avogadro’s number can be applied to compute various quantities that describe the composition of substances and. Web using avogadro’s number to convert from molecules or atoms to moles. Web the number 6.02 x 1023 is known as avogadro’s number in honor of an italian. Web a mole of objects contains avogadro's number, 6.022 x 1023, objects. Calculate the molar mass of calcium bromide. _____ b) 1 mole of water molecules contains how many h. Web the mole & avogadro's number. This number ( 6.02 x 1023) comes from the number of atoms in 12 g of. Web moles and molar mass. Using the information provided on slide #3, a) 1 mole of any substance equals how many of that substance? Download mole and avogadro’s number worksheet with answers and more chemistry exercises in pdf only on docsity! Make sure to learn these vocabulary terms and the kinds of chemical formulas they indicate. Just as a dozen. Web na ≈ 6.02 × 1023. Web view the mole and avogadros number worksheet key.pdf from bchs 4325 at university of houston. In chemistry, a mole is a really big number. Make sure to learn these vocabulary terms and the kinds of chemical formulas they indicate. What number of iron atoms, each weighing 55.847 u, is necessary to get 55.847. _____ b) 1 mole of water molecules contains how many h. Questions from the powerpoint lecture: _____ c) 1 mole of copper contains how many. A chemical species is either an atom (e.g. Web a mole of objects contains avogadro's number, 6.022 x 1023, objects. Download mole and avogadro’s number worksheet with answers and more chemistry exercises in pdf only on docsity! Make sure to learn these vocabulary terms and the kinds of chemical formulas they indicate. What number of iron atoms, each weighing 55.847 u, is necessary to get 55.847 g of fe? Apples, stars in the sky, burritos. Using the information provided on. Download mole and avogadro’s number worksheet with answers and more chemistry exercises in pdf only on docsity! Web the number 6.02 x 1023 is known as avogadro’s number in honor of an italian professor of physics, amadeo avogadro, who did considerable work on the development of atomic. Web the mole & avogadro's number. Web moles and molar mass. Web introducing. Web the mole & avogadro's number. Make sure to learn these vocabulary terms and the kinds of chemical formulas they indicate. Web partial preview of the text. Molar mass cabr2 = 199.886 g/mol 4. Using the information provided on slide #3, a) 1 mole of any substance equals how many of that substance? This number ( 6.02 x 1023) comes from the number of atoms in 12 g of. Molar mass cabr2 = 199.886 g/mol 4. So 6.02 × 1023 of what? Web using avogadro’s number to convert from molecules or atoms to moles. Web the mole & avogadro's number. Apples, stars in the sky, burritos. Web na ≈ 6.02 × 1023. In chemistry, a mole is a really big number. Using the information in the table, calculate the number of moles in a \pu {2.03 kg} 2.03 kg sample of citric acid ( \ce {c6h8o7} cx 6hx 8ox 7). Web the number 6.02 x 1023 is known as avogadro’s number in honor of an italian professor of physics, amadeo avogadro, who did considerable work on the development of atomic. A mole of iron atoms is 6.022 x 1023 iron atoms. This number ( 6.02 x 1023) comes from the number of atoms in 12 g of. Web view the mole and avogadros number worksheet key.pdf from bchs 4325 at university of houston. A chemical species is either an atom (e.g. Just as a dozen apples is 12 apples, a mole of apples is 6.022 x 1023 apples. Questions from the powerpoint lecture: Well, of anything you like: Molar mass cabr2 = 199.886 g/mol 4. Web avogadro’s number and the mole 1. Calculate the molar mass of calcium bromide. So 6.02 × 1023 of what? Download mole and avogadro’s number worksheet with answers and more chemistry exercises in pdf only on docsity! K, ag, s) , an ion (e.g. Web introducing moles and avogadro’s number. Define avogadro's number and explain why it is important to. Download mole and avogadro’s number worksheet with answers and more chemistry exercises in pdf only on docsity! Web moles and molar mass. In chemistry, a mole is a really big number. A mole of iron atoms is 6.022 x 1023 iron atoms. A mole of water molecules is 6.022 x 1023 water molecules. Web the mole & avogadro's number. So 6.02 × 1023 of what? Web partial preview of the text. Apples, stars in the sky, burritos. _____ b) 1 mole of water molecules contains how many h. Molar mass cabr2 = 199.886 g/mol 4. Web introducing moles and avogadro’s number. Just as a dozen apples is 12 apples, a mole of apples is 6.022 x 1023 apples. 2(79.904 g/mol) = 159.808 g/mol correct answer: Web the number 6.02 x 1023 is known as avogadro’s number in honor of an italian professor of physics, amadeo avogadro, who did considerable work on the development of atomic. Web avogadro’s number and the mole 1.The Mole And Avogadro's Number Worksheet Answers richinspire
The Mole And Avogadro's Number Worksheet Answers richinspire
Mole and Avogadro's Number Key
13 Best Images of Chemistry Mole Worksheet Mole Avogadro Number
Avogadro's Number And The Mole Worksheet
matter and measurement worksheet answers
39 the mole and avogadro's number worksheet answers Worksheet Master
14 Chemistry Mole Practice Worksheet /
The Mole And Avogadro's Number Worksheet Answers richinspire
Mole and Avogadro’s Number Worksheet with Answers Docsity
Web The Relationships Between Formula Mass, The Mole, And Avogadro’s Number Can Be Applied To Compute Various Quantities That Describe The Composition Of Substances And.
Make Sure To Learn These Vocabulary Terms And The Kinds Of Chemical Formulas They Indicate.
Using The Information Provided On Slide #3, A) 1 Mole Of Any Substance Equals How Many Of That Substance?
A Chemical Species Is Either An Atom (E.g.
Related Post: