The Mole And Avogadro's Number Worksheet
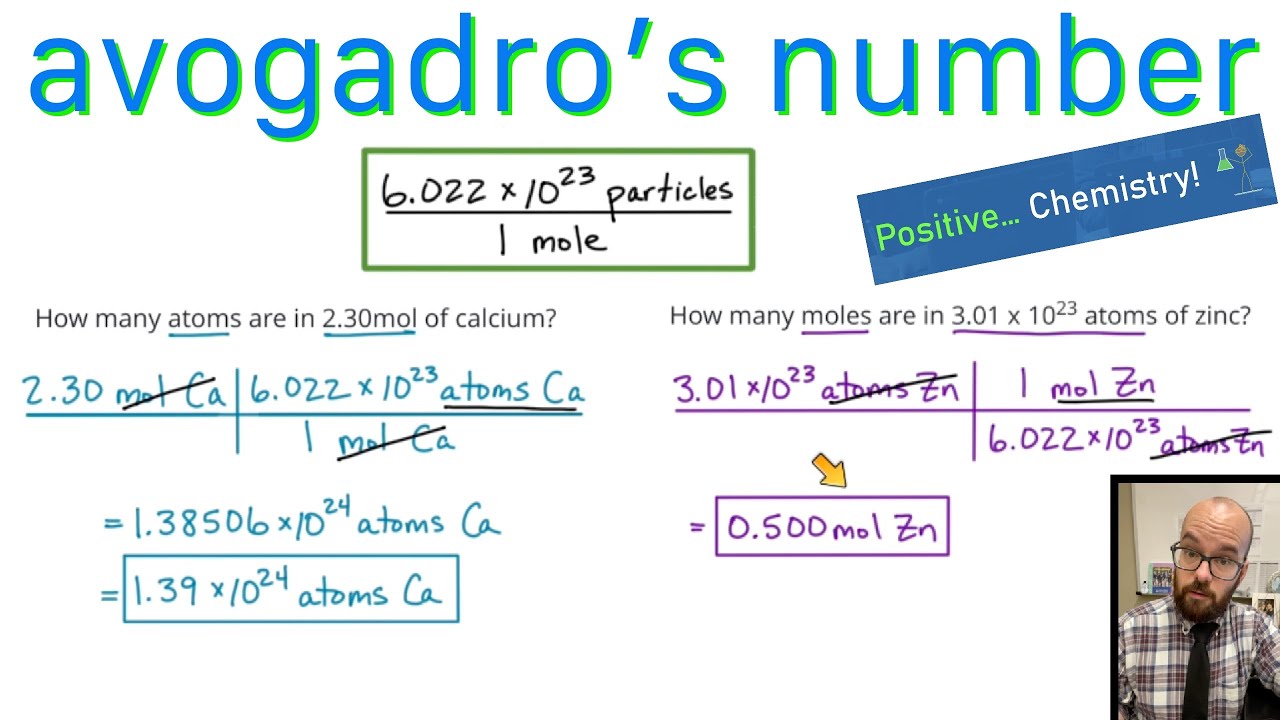
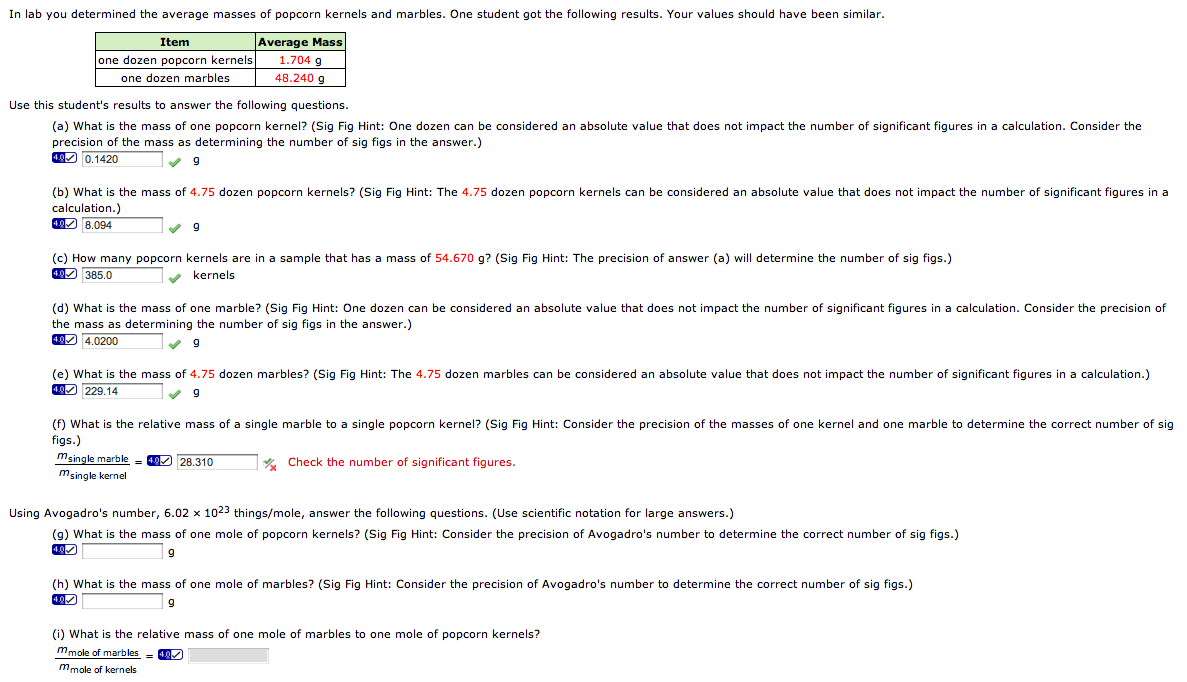
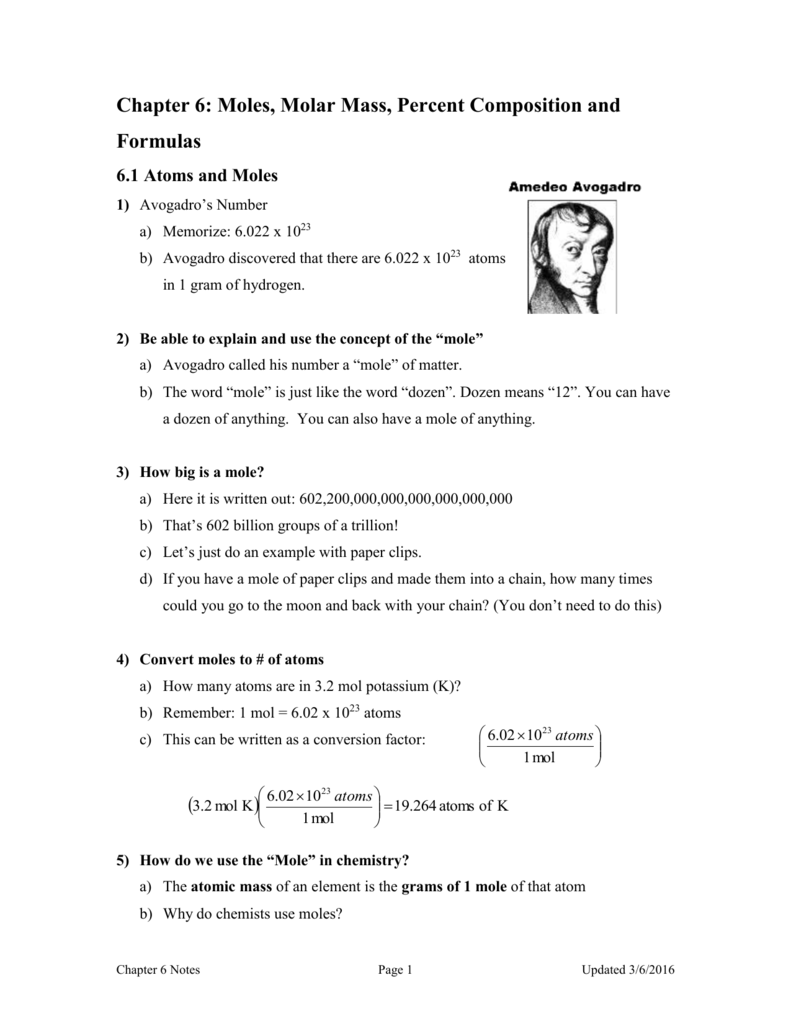
The Mole And Avogadro's Number Worksheet - Web introduction a mole of objects contains avogadro's number, 6.022 x 1023, objects. In chemistry, a mole is a really big number. Web moles and molar mass. A mole of objects contains avogadro's number, 6.022 x 1023, objects. Web avogadro’s number and the mole 1. Define molecular weight, formula weight, and molar mass; Web the mass of avogadro’s number of atoms is the atomic mass expressed in grams. Apples, a mole of apples is 6.022 x 1023 apples. Web view the mole and avogadros number worksheet key.pdf from bchs 4325 at university of houston. Mole of water molecules is. Web the relationships between formula mass, the mole, and avogadro’s number can be applied to compute various quantities that describe the composition of substances and. Web how many k+ ions? Worksheets are the mole and avogadros number, example exercise atomic mass and avogadros numb. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023. Mole of water molecules is. Now we have studied the idea of moles and learned three interpretations of a mole: Apples, a mole of apples is 6.022 x 1023 apples. Just as a dozen apples is 12 apples, a mole of apples is 6.022 x 1023 apples. Using the information in the table, calculate the number of moles in a. Just as a dozen apples is 12 apples, a mole of apples is 6.022 x 1023 apples. Web chemistry worksheet # 3 avogadro’s number one important property of a mole is that it means a definite number of particles just like a dozen means a number of particles. This is the definition of avogadro’s number. This number ( 6.02 x. Worksheets are the mole and avogadros number, example exercise atomic mass and avogadros numb. A) 152.3g hcn b) 1.3252 mol k2cro4 Elements generally exist as the particles we call atoms. Web introducing moles and avogadro’s number. Web the relationships between formula mass, the mole, and avogadro’s number can be applied to compute various quantities that describe the composition of substances. General review of mole problems. Determine the percent composition of each element in a compound from the chemical formula. Using the information in the table, calculate the number of moles in a \pu {2.03 kg} 2.03 kg sample of citric acid ( \ce {c6h8o7} cx 6hx 8ox 7). Web the mass of avogadro’s number of atoms is the atomic mass. This number ( 6.02 x 1023) comes from the number of atoms in 12 g of. Now we have studied the idea of moles and learned three interpretations of a mole: A mole of iron atoms is 6.022 x 1023 iron atoms. Calculate the empirical formula of a. Web view the mole and avogadros number worksheet key.pdf from bchs 4325. Web use avogadro's number to convert to moles and vice versa given the number of particles of an element. Just as a dozen apples is 12 apples, a mole of apples is 6.022 x 1023 apples. General review of mole problems. Web worksheet #13 using avogadro’s number and molar masses (2p.) answers. Web showing 8 worksheets for chemistry and avogadros. Know the definition of the mole. In chemistry, a mole is a really big number. Web convert between numbers of atoms, moles, and mass of sample by using avogadro’s number and the appropriate molar mass. Calculate the empirical formula of a. Determine the formula mass of an ionic or molecular compound. Explain how the latter differs from the first two. All answers have units of grams/mole agcl li2o fe(no3)3 143.32g/mol 29.88 241.88 cabr2199.88 c6h12o6 180.18 al2(so4)3 342.17 all answers to #2, #3 and #4 should have the chemical formula as part of the unit. This senior chemistry lesson package discusses the mole, avogadro’s number, molar mass and provides a lot of. Web how many k+ ions? Using the information in the table, calculate the number of moles in a \pu {2.03 kg} 2.03 kg sample of citric acid ( \ce {c6h8o7} cx 6hx 8ox 7). Worksheets are the mole and avogadros number, example exercise atomic mass and avogadros numb. Elements generally exist as the particles we call atoms. Web things to. In chemistry, a mole is a really big number. Just as a dozen apples is 12. Now we have studied the idea of moles and learned three interpretations of a mole: Web use avogadro's number to convert to moles and vice versa given the number of particles of an element. A mole of objects contains avogadro's number, 6.022 x 1023, objects. Determine the percent composition of each element in a compound from the chemical formula. Elements generally exist as the particles we call atoms. Web introducing moles and avogadro’s number. It is a huge number, far greater in magnitude than. Web the mass of avogadro’s number of atoms is the atomic mass expressed in grams. This number ( 6.02 x 1023) comes from the number of atoms in 12 g of. Therefore, 6.02 × 1023 atoms of (a) cu = 63.55 g (c) s = 32.07 g (b) hg = 200.59 g (d). Using the information in the table, calculate the number of moles in a \pu {2.03 kg} 2.03 kg sample of citric acid ( \ce {c6h8o7} cx 6hx 8ox 7). 1 mole of sucrose c12h22o11 contains 6.022 x 1023 molecules. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Web view the mole and avogadros number worksheet key.pdf from bchs 4325 at university of houston. Know the definition of the mole. Web the relationships between formula mass, the mole, and avogadro’s number can be applied to compute various quantities that describe the composition of substances and. Web how many k+ ions? A mole of iron atoms is 6.022 x 1023 iron atoms. Web chemistry worksheet # 3 avogadro’s number one important property of a mole is that it means a definite number of particles just like a dozen means a number of particles. Web view the mole and avogadros number worksheet key.pdf from bchs 4325 at university of houston. Web how many k+ ions? Web worksheet #13 using avogadro’s number and molar masses (2p.) answers. Just as a dozen apples is 12. Web introduction a mole of objects contains avogadro's number, 6.022 x 1023, objects. Therefore, 6.02 × 1023 atoms of (a) cu = 63.55 g (c) s = 32.07 g (b) hg = 200.59 g (d). All answers have units of grams/mole agcl li2o fe(no3)3 143.32g/mol 29.88 241.88 cabr2199.88 c6h12o6 180.18 al2(so4)3 342.17 all answers to #2, #3 and #4 should have the chemical formula as part of the unit. Know the definition of the mole. Determine the percent composition of each element in a compound from the chemical formula. This senior chemistry lesson package discusses the mole, avogadro’s number, molar mass and provides a lot of practice with the formulas to. Web the mass of avogadro’s number of atoms is the atomic mass expressed in grams. Elements generally exist as the particles we call atoms. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Web things to understand about avogadro's number. Web chemistry worksheet # 9:Mole and Avogadro’s Number Worksheet with Answers Docsity
The Mole And Avogadros Number Worksheet
The Mole And Avogadro's Number Worksheet pivotinspire
Avogadro’s Number Converting between atoms and moles YouTube
The Mole And Avogadro's Number Worksheet
The Mole And Avogadro's Number Worksheet
The Mole And Avogadro S Number Worksheet Answers Mole To Atom Atom
The Mole And Avogadro's Number Worksheet Answers richinspire
The Mole And Avogadro's Number Worksheet
The Mole And Avogadro's Number Worksheet pivotinspire
Web Introducing Moles And Avogadro’s Number.
Just As A Dozen Apples Is 12 Apples, A Mole Of Apples Is 6.022 X 1023 Apples.
Be Able To Find The Number Of Atoms Or Molecules In A Given Weight Of A Substance.
In Chemistry, A Mole Is A Really Big Number.
Related Post: