Thermochemical Equations Worksheet
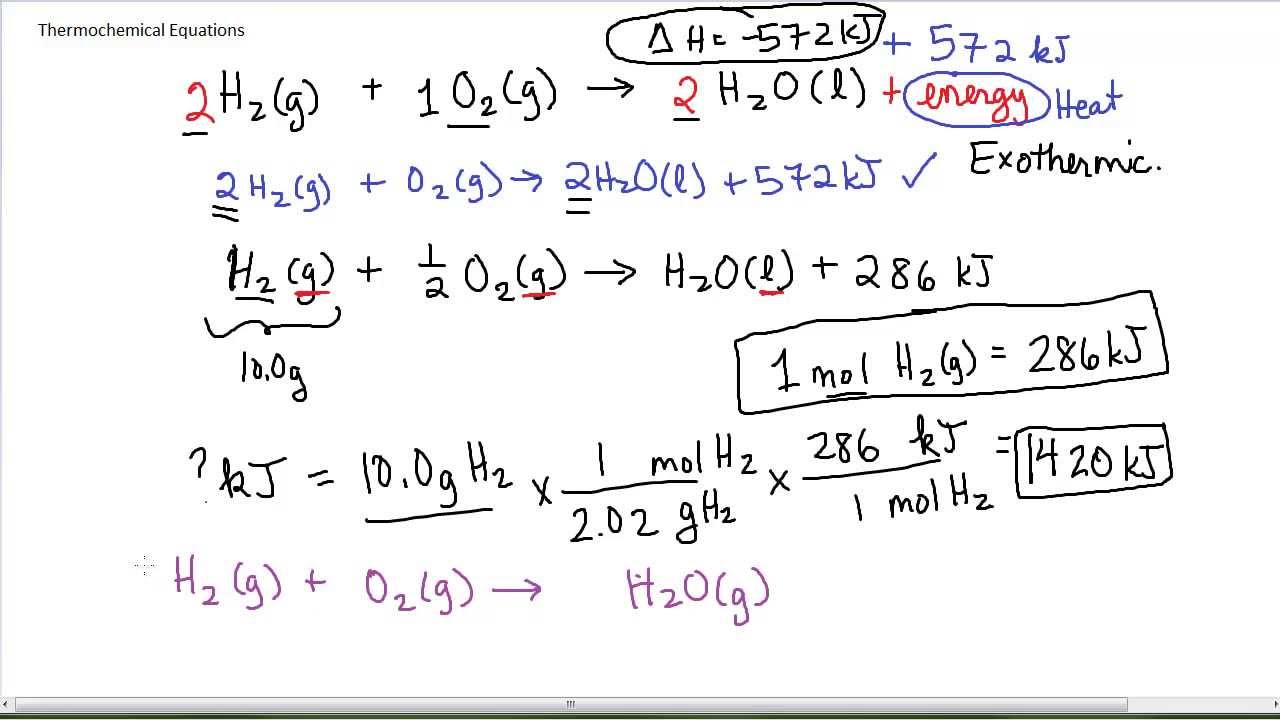
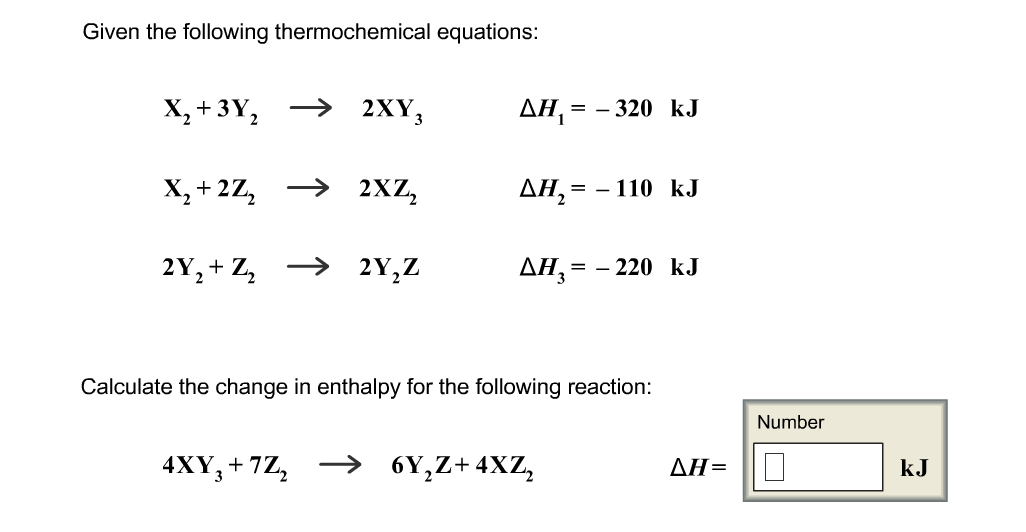
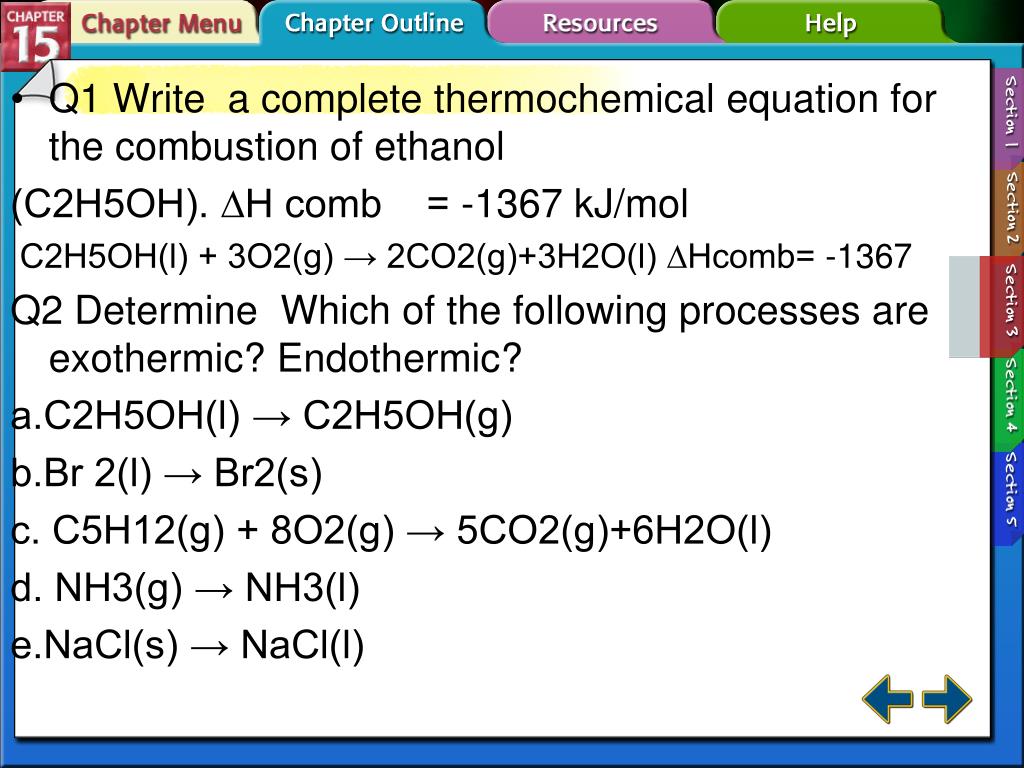
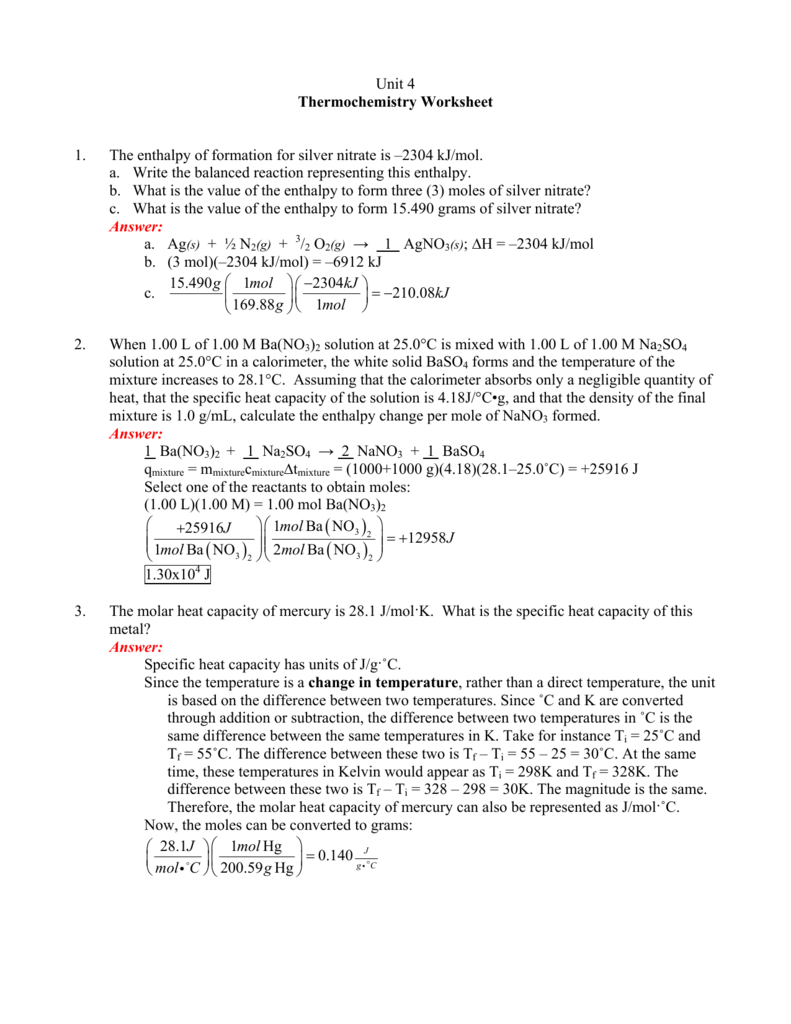
Thermochemical Equations Worksheet - Web thermochemical equations worksheet name: Kj/mol use heat of formation values. The enthalpy change for a reaction, ah, is negative. Write the balanced thermochemical equation. H2(g) + 4 o2(g) h h2o(l); Honors chemistry thermochemical equations practice worksheet 1. You must write all thermochemical equations for the steps of the cycle. The molar mass of ethane is 28.0 g/mol. How many grams of iron will be produced when 54.0 j of heat are absorbed? Web rules for using thermochemical equations. What does this indicate about the chemical potential energy of the system before and after the reaction? Thermochemistry 165 substituting into the equation derived above, we can solve for tf. Δh = + 26.3 kj. You must be able to explain different types of reactions and know. The enthalpy change for a reaction, ah, is negative. Web a thermochemical equation gives the enthalpy change for the quantities of reactants and products (in moles) in the specified physical states. Thermochemistry 165 substituting into the equation derived above, we can solve for tf. Web rules for using thermochemical equations. This quiz aligns with the following ngss standard (s): Web write the balanced thermochemical equation for the heat of. How is a thermochemical equation different from a balanced chemical equation? How many grams of iron will be produced when 54.0 j of heat are absorbed? 2f2(g) + 2h2o(l) 4hf(g) + o2(g) h = ? Web describe the following processes as exothermic or endothermic: Web write the balanced thermochemical equation for the heat of combustion of benzene. Web rules for using thermochemical equations. Web when manipulating thermochemical equations, if i divide the coefficients of the chemical reaction of one step by 3, then what do i need to do to the enthalpy change value? Web write the balanced thermochemical equation for the heat of combustion of benzene. Web describe the following processes as exothermic or endothermic: Web. Web we can easily calculate the molar mass of octane: Kj/mol use heat of formation values. Must the final temperature be between the two starting values? Co(g) + sio2(s) ( sio(g) + co2(g) (h = +520.9 kj. 1.000g c8h18 x 1 mole x 10,148 kj = 44.41 kj 114.26g 2 moles c8h18. Is this reaction exothermic or endothermic? How much heat is absorbed for the production of 6.5 g of iron (iii) oxide? Honors chemistry thermochemical equations practice worksheet 1. +6 kj) the complete combustion of liquid octane, c 8 h 18 , produces carbon dioxide and water at 25 oc and at constant pressure, it gives 47 kj of heat per. Is this reaction exothermic or endothermic? [usually for 25°c (298 k) and 1 atm]. Calculate the heat of the reaction for the following reaction: Based on your answer to question ii, write an expression for the heat of combustion of benzene, \(δh^o_{comb}\), in terms of the enthalpies of formation of the reactants and products. When one mole of ice melts,. Web rules for using thermochemical equations. This quiz aligns with the following ngss standard (s): What does this indicate about the chemical potential energy of the system before and after the reaction? How many grams of iron will be produced when 54.0 j of heat are absorbed? Web use the following equations and data. H2(g) + 4 o2(g) h h2o(l); H 2 o (s) h 2 o (l) d h = 6.00 kj. Web use the following equations and data. Web we can easily calculate the molar mass of octane: Cao(s) + so3(g) ( caso4(s) (h = ? The enthalpy change for a reaction, ah, is negative. Web thermochemical equations worksheet name: Must the final temperature be between the two starting values? Web a thermochemical equation gives the enthalpy change for the quantities of reactants and products (in moles) in the specified physical states. Web this quiz/worksheet assessment tool has been designed to help you quickly gauge your. Web thermochemical equations rule #1 • ∆h is directly proportional to the amount of reactants and products. Is this reaction exothermic or endothermic? Web thermochemical equations worksheet name: Use the following data to calculate the lattice energy of cesium oxide. 8co2(g) + si3n4(s) ( 3sio2(s) + 2n2o(g) + 8co(g) (h = +461.05 kj. Web a thermochemical equation gives the enthalpy change for the quantities of reactants and products (in moles) in the specified physical states. H 2 o (s) h 2 o (l) d h = 6.00 kj. Use the following equations and data. What does this indicate about the chemical potential energy of the system before and after the reaction? Web we can easily calculate the molar mass of octane: How is a thermochemical equation different from a balanced chemical equation? Honors chemistry thermochemical equations practice worksheet 1. +6 kj) the complete combustion of liquid octane, c 8 h 18 , produces carbon dioxide and water at 25 oc and at constant pressure, it gives 47 kj of heat per gram of octane. This online quiz is intended to give you extra practice in performing thermochemical calculations with a variety of reactions, including limiting reagents and percent yield options. Ammonium nitrate is mixed in water, resulting in a very cold solution. Thermochemistry 165 substituting into the equation derived above, we can solve for tf. How many grams of iron will be produced when 54.0 j of heat are absorbed? Equations add to my workbooks (0) download file pdf embed in my website or blog add to google classroom Based on your answer to question ii, write an expression for the heat of combustion of benzene, \(δh^o_{comb}\), in terms of the enthalpies of formation of the reactants and products. Calculate the amount of heat released when 4.79 g of c2h4 reacts with excess oxygen. H 2 o (s) h 2 o (l) d h = 6.00 kj. Use the following equations and data. The combustion of ethane (c2h4) is an exothermic reaction. Web a thermochemical equation gives the enthalpy change for the quantities of reactants and products (in moles) in the specified physical states. How is a thermochemical equation different from a balanced chemical equation? Web use the following equations and data. Use the following data to calculate the lattice energy of cesium oxide. Cao(s) + so3(g) ( caso4(s) (h = ? 8co2(g) + si3n4(s) ( 3sio2(s) + 2n2o(g) + 8co(g) (h = +461.05 kj. The molar mass of ethane is 28.0 g/mol. Web this quiz/worksheet assessment tool has been designed to help you quickly gauge your understanding of thermochemical equations. Web write the balanced thermochemical equation for the heat of combustion of benzene. Co(g) + sio2(s) ( sio(g) + co2(g) (h = +520.9 kj. How much heat is absorbed for the production of 6.5 g of iron (iii) oxide? Δh = + 26.3 kj. How many grams of iron will be produced when 54.0 j of heat are absorbed?thermochemical equations worksheet
Thermochemical Equations YouTube
Thermochemistry Worksheet
Worksheet Thermochemistry I Answers
Thermochemical Equations YouTube
thermochemical equations worksheet
thermochemical equations worksheet
thermochemical equation worksheet
Quiz & Worksheet Thermochemical Equations
Writing "Thermochemical" Equations Worksheet for 9th Higher Ed
+6 Kj) The Complete Combustion Of Liquid Octane, C 8 H 18 , Produces Carbon Dioxide And Water At 25 Oc And At Constant Pressure, It Gives 47 Kj Of Heat Per Gram Of Octane.
What Does This Indicate About The Chemical Potential Energy Of The System Before And After The Reaction?
2F2(G) + 2H2O(L) 4Hf(G) + O2(G) H = ?
You Must Write All Thermochemical Equations For The Steps Of The Cycle.
Related Post: