Thermochemistry Review Worksheet
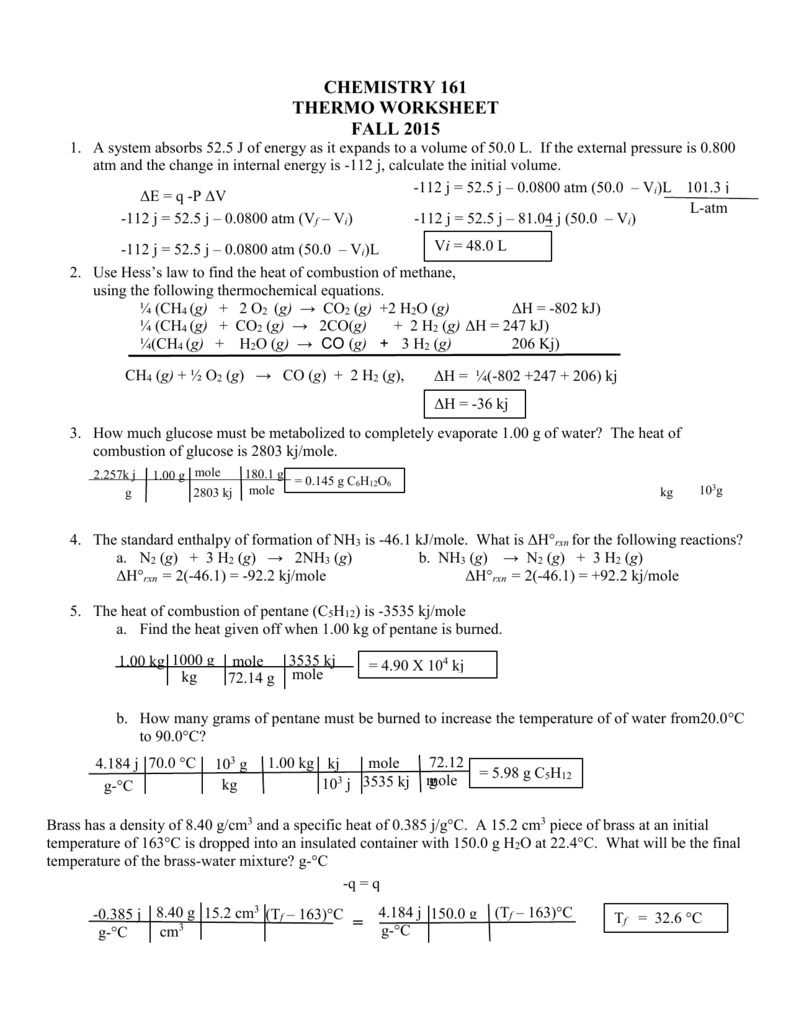
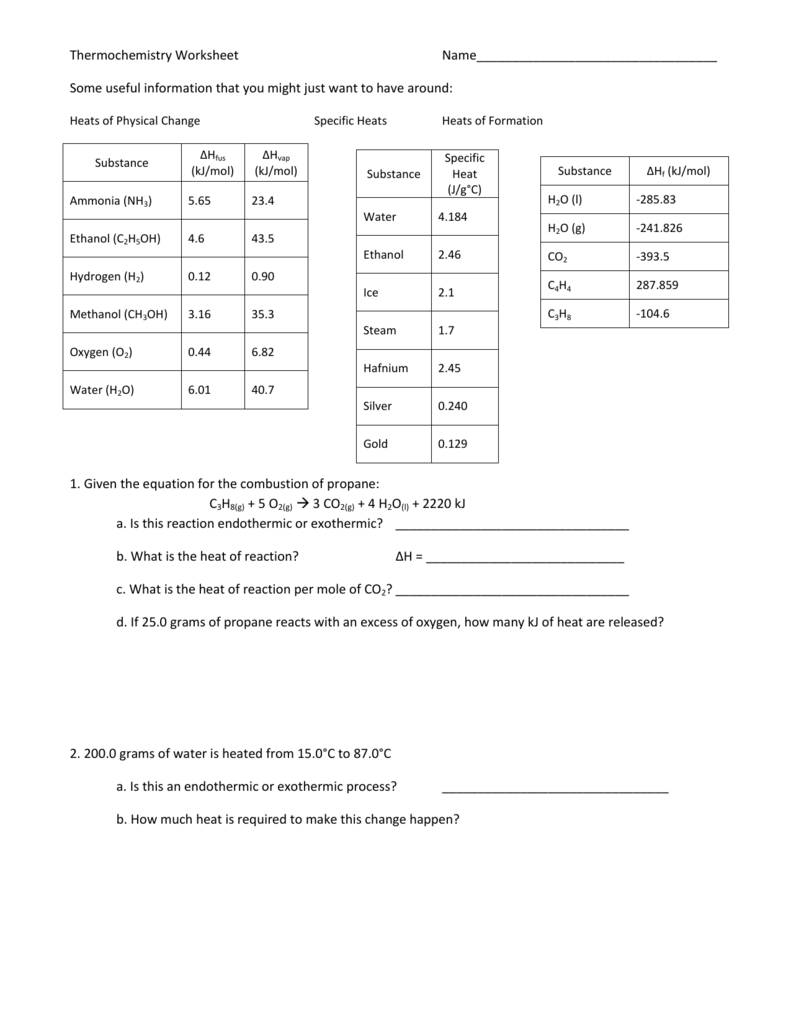
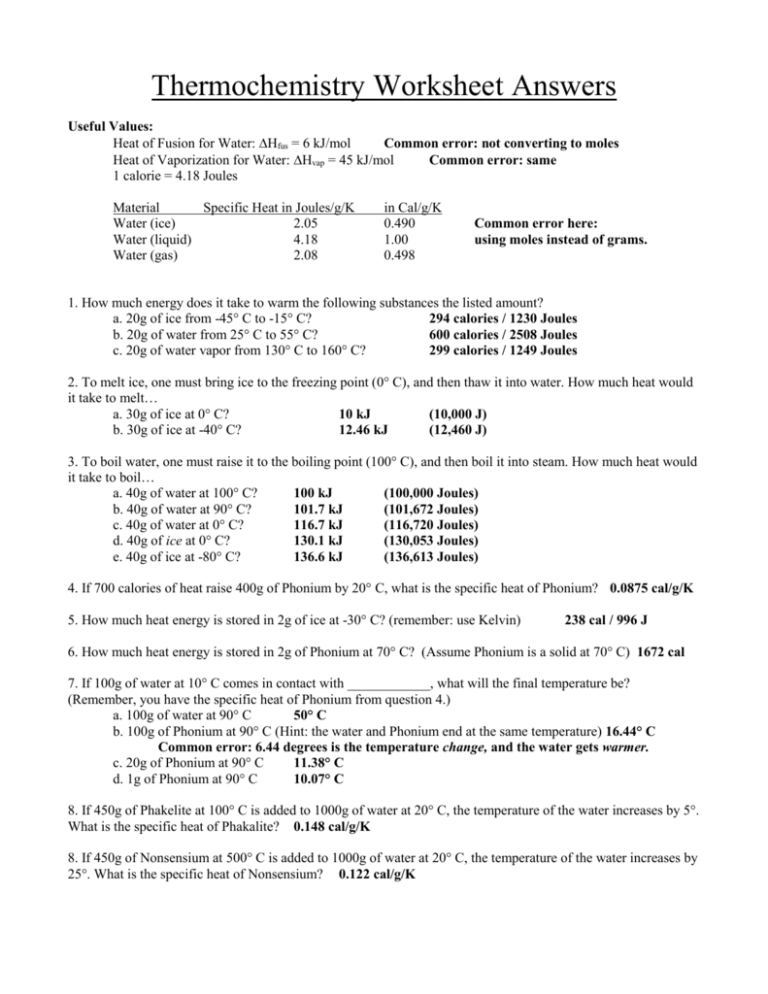
Thermochemistry Review Worksheet - 3) describe what we mean by. Calculate the heat of the reaction for the following reaction: _____ when we mix water at 100 c with water at 20 c the final water temp. _____ when we put a 100g piece of metal at 100 c into 100g of water at 20 c the final temp is 20 c. Final discussion for hesi rn. W = f × d. Web consider the thermochemical equation below: (s) + h2o(g) co + h. A piece of aluminum with a mass of 50g and an initial temperature of 90oc is placed into 100ml of water at a temperature of 25oc. Web (choice a) at atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute zero. _____ chemical energy is a form of kinetic energy. Fill out the empty areas; Web consider the thermochemical equation below: The specific heat capacity of diamond is 0.5050 j/gºc. Web 8 worksheets final exam review worksheets grade 11 chemistry with answers. Please sign inor registerto post comments. Calculate &𝗃 for the overall process. How many joules of heat energy would be required to raise the temperature of 16.0g of lead from 25˚c to its melting point of 327˚c for a length of time long enough to completely melt the lead. Web thermochemistry review quiz for 10th grade students. &𝗃 = 𝗅. Heat lost by system to surroundings is _________________. How much energy is required to heat 25.0 g of diamond from 10.5ºc to 15.6ºc? Involved parties names, addresses and numbers etc. 3) describe what we mean by. A piece of aluminum with a mass of 50g and an initial temperature of 90oc is placed into 100ml of water at a temperature. The worksheets include answers and combined they are 27 pages long. 4 no (g) + 6 h 2 o (l) → 4 nh 3 (g) + 5 o2 (g) ∆rh° = 1170 kj. Give at least 3 examples of each that you encounter in everyday life. This is a final exam review, for grade 11 chemistry that contains 8 different. Organic chemistry i (chem 624) academic year:2016/2017. Classify each of these reactions as exothermic or endothermic: True or false if the answer is false, you must explain why. 4 no (g) + 6 h 2 o (l) → 4 nh 3 (g) + 5 o2 (g) ∆rh° = 1170 kj. When a 7 g sample of sulfur (s 8 ). Web the heat capacity, c, is the amount of heat, q, required to raise the temperature, δ t, of an object by 1 o c. Give at least 3 examples of each that you encounter in everyday life. Web short answer / problems: The system absorbs 35 j of heat while performing. Web general chemistry i (141) thermochemistry worksheet answers. True or false if the answer is false, you must explain why. Symbolized by f, any kind of push or pull exerted on an object. A complete answer key is provided at the end. Web thermochemistry review quiz for 10th grade students. 2) what is the difference between heat and temperature? Symbolized by f, any kind of push or pull exerted on an object. The specific heat capacity of lead is 0.159j/gk and the molar enthalpy of fusion is 24.7j/g. (choice b) solid and gaseous helium never exist in equilibrium with each other at any temperature or pressure. (g)2(g) h2o(l) + 40.7 kj h2o(g). Web thermochemistry worksheets and online activities. The value of c in this equation, and likewise the magnitudes of q and δ t, pertain to a certain sample and depend on the amount. Web the heat capacity, c, is the amount of heat, q, required to raise the temperature, δ t, of an object by 1 o c. 2c2h2(g) + 5/o2(g) 4co2(g) + 2h2o(g) h = ?. Where w is work, f is force, and d is distance that the object is being moved. 1.9 x 102 kj 4. &𝗃 = 𝗅 + 𝗆 = 72𝗃 + 35𝗃. Web 8 worksheets final exam review worksheets grade 11 chemistry with answers. Symbolized by w, movement of an object against some force. Please sign inor registerto post comments. Energy that is transferred from one object to another because of difference in temperature. The system absorbs 72 j of heat while 35 j of work is being done on it. A complete answer key is provided at the end. The specific heat capacity of lead is 0.159j/gk and the molar enthalpy of fusion is 24.7j/g. Classify each of these reactions as exothermic or endothermic: Web consider the thermochemical equation below: (s) + h2o(g) co + h. When a 7 g sample of sulfur (s 8 ) is burned in a bomb calorimeter with excess oxygen, the temperature increases from 20 °c to 36 °c. Symbolized by w, movement of an object against some force. The system absorbs 35 j of heat while performing. The specific heat capacity of diamond is 0.5050 j/gºc. Web chapter 10 practice worksheet: True or false if the answer is false, you must explain why. Web find the thermochemistry review worksheet answers you require. This is a final exam review, for grade 11 chemistry that contains 8 different worksheets. Calculate &𝗃 for the overall process. 4 no (g) + 6 h 2 o (l) → 4 nh 3 (g) + 5 o2 (g) ∆rh° = 1170 kj. How many joules of heat energy would be required to raise the temperature of 16.0g of lead from 25˚c to its melting point of 327˚c for a length of time long enough to completely melt the lead. Change the blanks with smart fillable areas. Pcl + cl pcl3(l) + energy 2(g)5(g) c. Calculate &𝗃 for the overall process. _____ chemical energy is a form of kinetic energy. Please sign inor registerto post comments. Web short answer / problems: A piece of aluminum with a mass of 50g and an initial temperature of 90oc is placed into 100ml of water at a temperature of 25oc. 3) describe what we mean by. How many joules of heat energy would be required to raise the temperature of 16.0g of lead from 25˚c to its melting point of 327˚c for a length of time long enough to completely melt the lead. 2c2h2(g) + 5/o2(g) 4co2(g) + 2h2o(g) h = ? Where w is work, f is force, and d is distance that the object is being moved. Involved parties names, addresses and numbers etc. Web consider the thermochemical equation below: Include the day/time and place your electronic signature. This is a final exam review, for grade 11 chemistry that contains 8 different worksheets. Web thermochemistry (worksheet) last updated. Final discussion for hesi rn.review for thermochemistry test
Thermochemistry Worksheet
Thermochemistry Review Worksheet Key kidsworksheetfun
Thermochemistry worksheet 1 Key
Thermochemistry Review Worksheet
Ultimate Thermochemistry Worksheet
Thermochemistry Review Worksheet1
Thermochemistry Worksheet Answers
Thermochemistry Review Worksheet Key kidsworksheetfun
Thermochemistry Worksheet
The Value Of C In This Equation, And Likewise The Magnitudes Of Q And Δ T, Pertain To A Certain Sample And Depend On The Amount.
Web Chapter 10 Practice Worksheet:
Web Thermochemistry Worksheets And Online Activities.
(S) + H2O(G) Co + H.
Related Post: