Thermochemistry Worksheet Answers
Thermochemistry Worksheet Answers - What is the final temperature of the part? Worksheets are thermochemistry, thermochemistry, thermochemistrypractice thermochemical equations and, thermochemistry calculations work 1, ap chemistry review work unit 4, answers thermochemistry practice problems 2, , chapter 17 thermochemistry work. What is an exothermic reaction? Calculate the heat of the reaction for the following reaction: ( c of al = 2.42 j/g * k) q2. (explain what happens in terms of heat flow, and what happens to the temperature of the surroundings). A detailed edit history is available upon request. (include a definition and it’s symbol). Q = m × c × δt. When the reaction occurs, heat is released, stabilizing the system and the system moves to a lower energy potential. Web 1) describe the difference between potential energy and kinetic energy. Hess's law and reaction enthalpy change. Q = m × c × δt. Embed in my website or blog. Give at least 3 examples of each that you encounter in everyday life. (notice the conversion to j.). A 295 g aluminum engine part at an initial temperature of 3.00°c absorbs 85.0 kj of heat. Web the reaction is endothermic. More practice with calorimetry problemsws3: 3) describe what we mean by conservation of energy. Give at least 3 examples of each that you encounter in everyday life. Hc 2h 3o 2(l),+ 2o 2(g)! Heat lost by system to surroundings is _________________. How much heat, in calories, is released or absorbed if a 15 g sample of copper is converted from (a) the solid to liquid state. Over 100°c, h2o will be a gas.) 2. Calculate the energy associated with this reaction. Gibbs free energy and spontaneity. (explain what happens in terms of heat flow, and what happens to the temperature of the surroundings). (notice the conversion to j.). Worksheets are thermochemistry, thermochemistry, thermochemistrypractice thermochemical equations and, thermochemistry calculations work 1, ap chemistry review work unit 4, answers thermochemistry practice problems 2, , chapter 17. Web the reaction is endothermic. Final discussion for hesi rn. Because the si unit of force, the newton, is defined as. Web 141 thermochemistry worksheet key. A 295 g aluminum engine part at an initial temperature of 3.00°c absorbs 85.0 kj of heat. Q = (120.0 g) (22.0 °c) (4.184 j/g °c) = 11000 j. We will ignore any heats losses to the walls of the container and losses to the air. 2) what is the difference between heat and temperature? What is an endothermic reaction? When the reaction occurs, heat is released, stabilizing the system and the system moves to a lower. Heat lost by system to surroundings is _________________. ( c of al = 2.42 j/g * k) q2. Q = m × c × δt. Embed in my website or blog. Use heat of formation values. Calculate the heat of the reaction for the following reaction: Classify each of these reactions as exothermic or endothermic: 3) describe what we mean by conservation of energy. When the reaction occurs, heat is released, stabilizing the system and the system moves to a lower energy potential. (choice a) at atmospheric pressure, helium can exist in all three phases, as. We will ignore any heats losses to the walls of the container and losses to the air. (choice a) at atmospheric pressure, helium can exist in all three phases, as well as a supercritical fluid phase near absolute. Web but notice that the answer we obtain here is the following sum, where δ hof (h 2) = 0, because h. Use heat of formation values. Web 141 thermochemistry worksheet key. When the reaction occurs, heat is released, stabilizing the system and the system moves to a lower energy potential. General chemistry i (141) thermochemistry worksheet answers. 4) draw a picture showing the direction of heat flow in an endothermic reaction versus an exothermic reaction. Q = (120.0 g) (22.0 °c) (4.184 j/g °c) = 11000 j. The reactant is at a higher potential than the product. Q = (20.0 g) (20.0 °c) (2.02 j/g °c). What is the final temperature of the part? Is this reaction exothermic or endothermic? General chemistry i (141) thermochemistry worksheet answers. Because the si unit of force, the newton, is defined as. {\rm { = }}\;\frac { {\rm {q}}} { { {\rm {m}}\; Δho rxn = δho f(c 2h 4) − [δho f(c 2h 2) + δho f(h 2)] = + 52.3kj − [ − 226.8kj + 0kj] Web the si unit of energy is the joule (j), which is a derived unit defined as follows: 4) draw a picture showing the direction of heat flow in an endothermic reaction versus an exothermic reaction. Web 1) describe the difference between potential energy and kinetic energy. Heat lost by system to surroundings is _________________. What is an endothermic reaction? A 295 g aluminum engine part at an initial temperature of 3.00°c absorbs 85.0 kj of heat. (b) the gas to the liquid state. ∆hfus = 206 j/g, ∆hvap = 4 kj/g This bundle of 10 worksheets cover the following thermochemistry topics: Classify each of these reactions as exothermic or endothermic: Gibbs free energy and spontaneity. The reactant is at a higher potential than the product. This bundle of 10 worksheets cover the following thermochemistry topics: More practice with calorimetry problemsws3: 800,000 j = (720.0 g) (x) (2.02 j/g °c). Add to my workbooks (10) download file pdf. Newton(n) ≡ kg × m s2. These is a typical position to take since, in a real experiment, both would have to be accounted for, making for much more complexity. Q = (120.0 g) (22.0 °c) (4.184 j/g °c) = 11000 j. > thermochemistry worksheet + answers. What is the final temperature of the part? The reaction of magnesium with sulfuric acid was carried out in a calorimeter. Q = m × c × δt. We will ignore any heats losses to the walls of the container and losses to the air. (include a definition and it’s symbol). (notice the conversion to j.). Calculate the energy associated with this reaction.Thermochemistry Worksheet 1 Answers Promotiontablecovers
30 Thermochemistry Problems Worksheet Number One Answers support
Thermochemistry Worksheet Answers
thermochemistry worksheet with answers
Thermochemistry Calorimetry Worksheet Answers Reporteral
Thermochemistry Worksheet 2 With Answers Printable Worksheets For
enthalpy worksheet with answers
️Thermochemistry Worksheet Answers Free Download Qstion.co
Thermochemistry Review Answers1
Thermochemistry Worksheet 1 Answers Promotiontablecovers
What Is An Exothermic Reaction?
Joule(J) ≡ Kg × M2 S2.
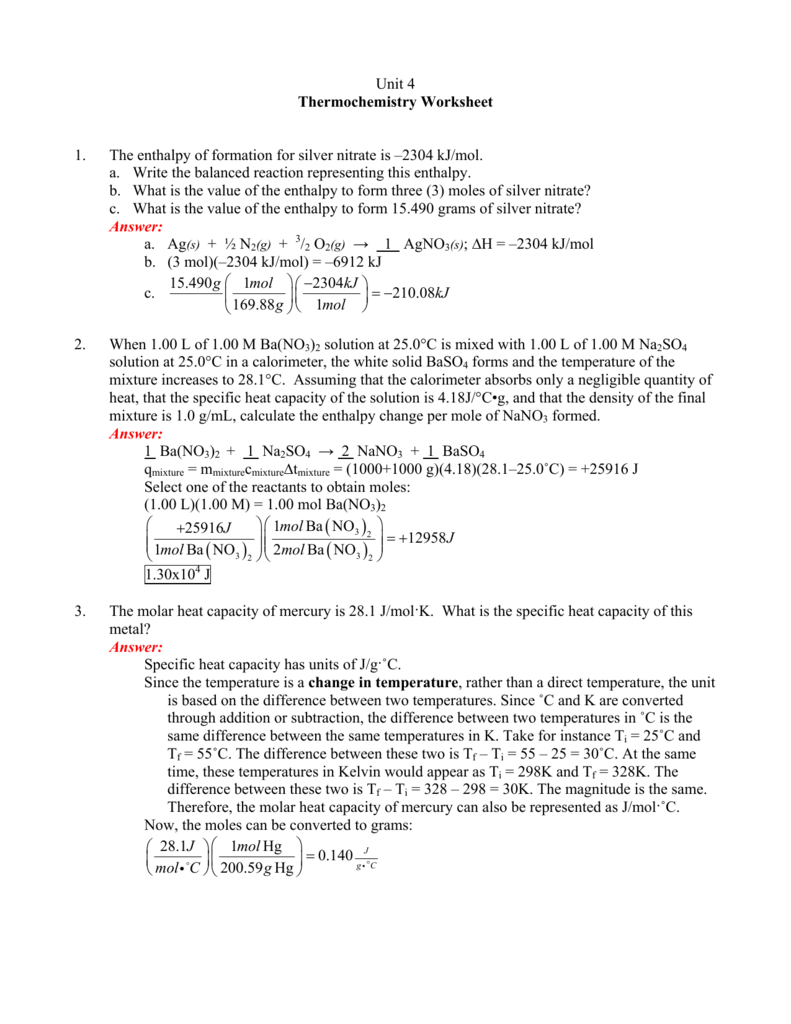
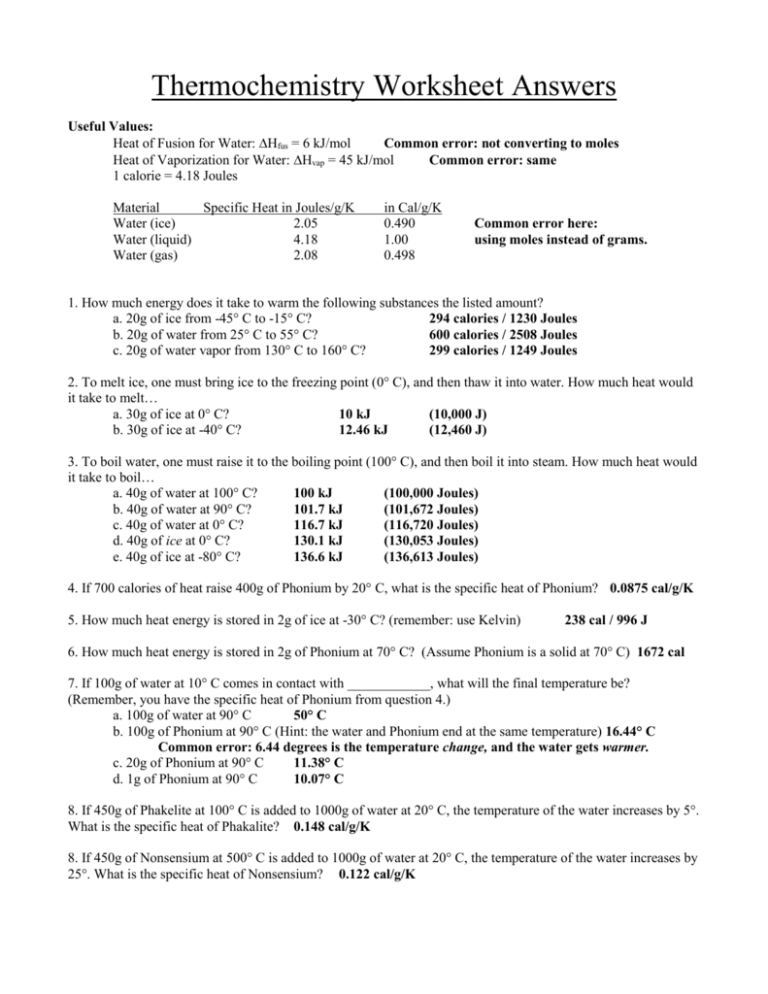
Calculate The Heat Of The Reaction For The Following Reaction:
Use Heat Of Formation Values.
Related Post: