Vsepr Theory Worksheet
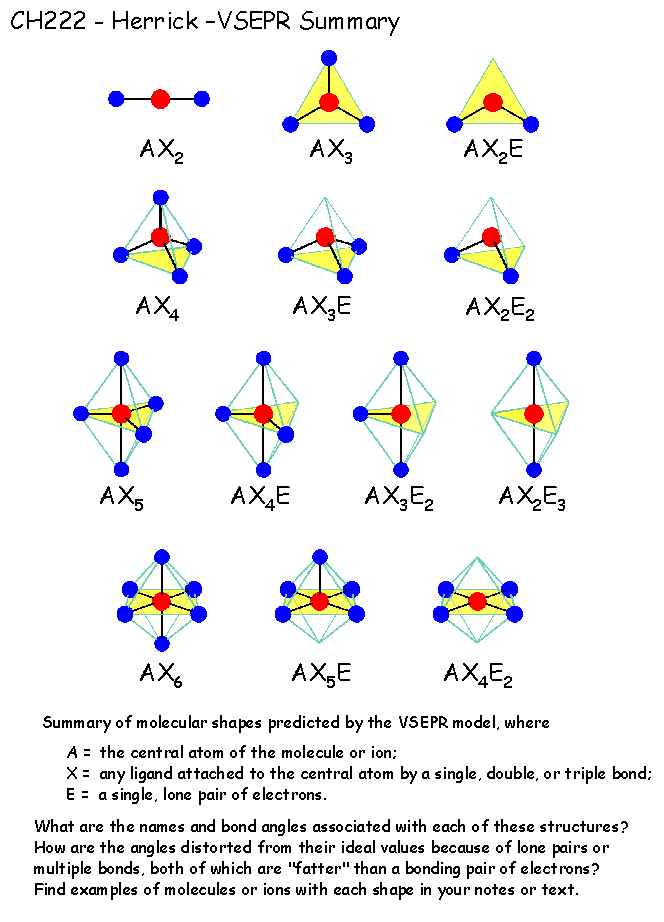
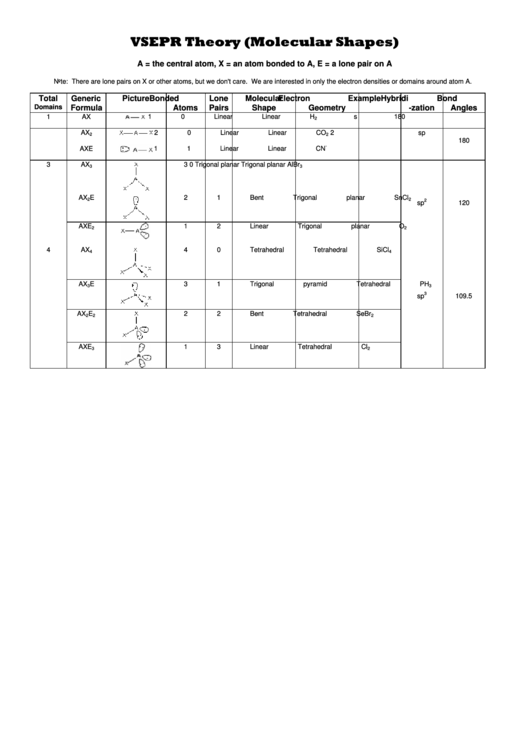
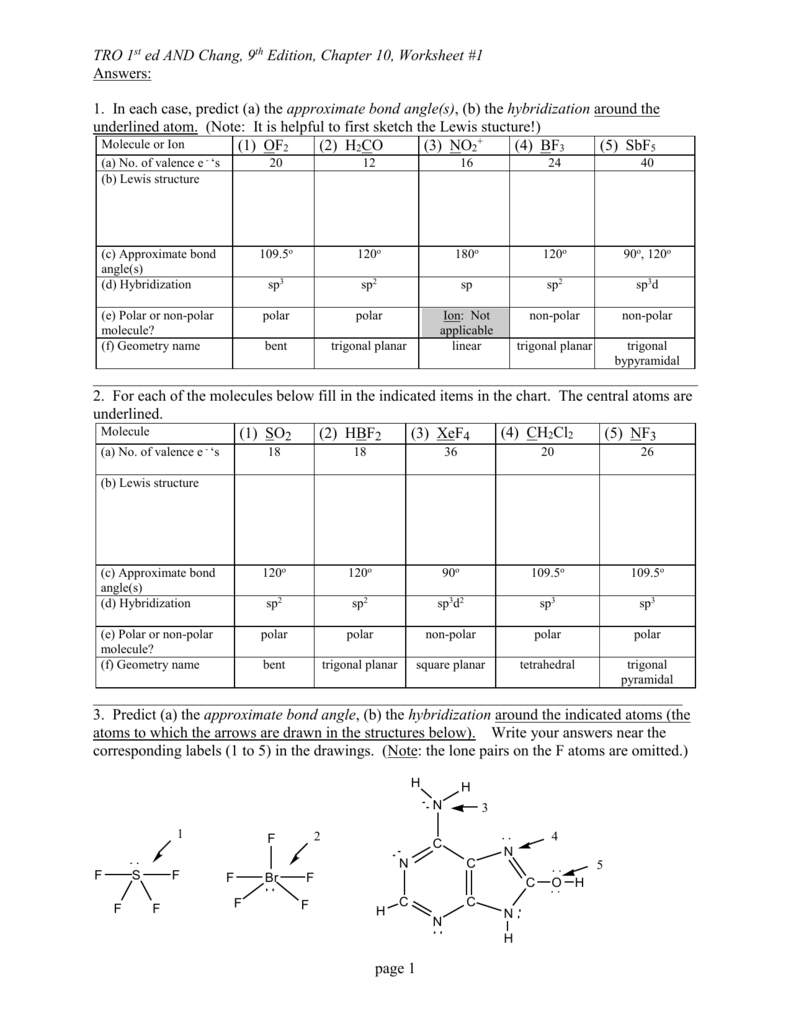
Vsepr Theory Worksheet - Web sch4u1 molecular geometry and vsepr name: Web vsepr theory interactive and downloadable worksheets. Notes there are no stable axe 4, ax 3e 3, ax 2e 4 5 or axe molecules. A compounds 1 and 2 are both polar. 2) for each of the following compounds, a lewis structure, determine the bond angles and molecular shapes for all atoms: Web vsepr practice for each of the following compounds, identify the bond type in the first column, draw the (lewis) dot structure / electron dot formula in the second column. The molecules are of the abn type with a central atom from the p block of the periodic table. Hybridization and resonance structures identify: A) bi 3 b) ch 4 c) nf 3 d) c 2 h 2 e) scl 6 Then, if the compound is covalent, identify the number of shared electrons pairs on the central atom, # of unshared pairs (lone pairs), and the correct the molecular shape. Compound 1 is nonpolar, whereas compound 2 is polar. Determine the electron geometry and molecular geometry of the following molecules using the vsepr model. Hybridization and resonance structures identify: There are lone pairs on x or other atoms, but we don't care. For each of the following compounds, determine the bond angles, molecular shapes, and hybridizations for all atoms: As a result, the atoms in a molecule tend to separate as far as they can because their bonds repel each other. Web explore molecule shapes by building molecules in 3d! Web vsepr theory states that regions of high electron density, such as bonding pairs or lone pairs of electrons (a vsepr or electron domain), will arrange themselves as far. Web vsepr worksheet 1) what is the main idea behind vsepr theory? Compound 1 is polar, whereas compound 2 is nonpolar. The molecules are of the abn type with a central atom from the p block of the periodic table. Compounds 1 and 2 are both nonpolar. Web with this worksheet packet, your chemistry students will receive essential practice on. Web vsepr theory states that regions of high electron density, such as bonding pairs or lone pairs of electrons (a vsepr or electron domain), will arrange themselves as far apart as possible around the central atom. Web sch4u1 molecular geometry and vsepr name: Web students must use vsepr theory and electrthis bundle contains worksheets for teaching an introduction to ionic. Web 1) what is the main idea behind vsepr theory? 2) for each of the following compounds, determine the bond angles, molecular shapes, and hybridizations for all atoms: Linear, trigonal planar, pyramid, bent (angular), tetrahedral, and trigonal bipyramidal. Web explore molecule shapes by building molecules in 3d! Determine the central atom and draw the lewis structure for the molecule. C compound 1 is polar, whereas compound 2 is nonpolar. Web vsepr worksheet 1) what is the main idea behind vsepr theory? A) answer follow these steps to determine the electron and molecular geometries: A compounds 1 and 2 are both polar. Web vsepr theory (molecular shapes) = the central atom, x = an atom bonded to a, e =. What is the main idea behind vsepr theory? This resource includes 7 one page worksheets with spaces for student answers as well as. There are lone pairs on x or other atoms, but we don't care. For electron pairs (base shape) Then, if the compound is covalent, identify the number of shared electrons pairs on the central atom, # of. Web vsepr theory (molecular shapes) = the central atom, x = an atom bonded to a, e = a lone pair on a note: Web explore molecule shapes by building molecules in 3d! Hybridization and resonance structures identify: Then, compare the model to real molecules! Web vsepr worksheet 1) briefly describe the primary ideas behind vsepr theory. The molecules are of the abn type with a central atom from the p block of the periodic table. The main idea is that electrons don’t like to hang around near each other because they repel each other. What is the main idea behind vsepr theory? Web the vsepr (valence shell electron pair repulsion) model, which states that electron pairs. Web with this worksheet packet, your chemistry students will receive essential practice on the shapes of molecules and ions by applying the vsepr theory. C compound 1 is polar, whereas compound 2 is nonpolar. Determine the electron geometry and molecular geometry of the following molecules using the vsepr model. Web the vsepr (valence shell electron pair repulsion) model, which states. 2) for each of the following compounds, a lewis structure, determine the bond angles and molecular shapes for all atoms: Web vsepr theory (molecular shapes) = the central atom, x = an atom bonded to a, e = a lone pair on a note: Compounds 1 and 2 are both polar. Are all five possible electronic geometries represented in this set of molecules? Find out by adding single, double or triple bonds and lone pairs to the central atom. Web vsepr practice for each of the following compounds, identify the bond type in the first column, draw the (lewis) dot structure / electron dot formula in the second column. The molecules are of the abn type with a central atom from the p block of the periodic table. 2) for each of the following compounds, determine the bond angles, molecular shapes, and hybridizations for all atoms: Hybridization and resonance structures identify: A compounds 1 and 2 are both polar. Bi3 ch4 nf3 c2h2 everett community college tutoring center student support services program Web students must use vsepr theory and electrthis bundle contains worksheets for teaching an introduction to ionic and covalent compounds, naming and formula writing for simple binary ionic compounds, ionic compounds with polyatomic ions, ionic compounds with transition metals, covalent compounds, and a bonus activity for simple vsepr. For electron pairs (base shape) For each of the following compounds, a lewis structure, determine the bond angles and molecular shapes for all atoms: This resource includes 7 one page worksheets with spaces for student answers as well as. G e n e ra l f o rmu l. Determine the electron geometry and molecular geometry of the following molecules using the vsepr model. If not, which ones are missing? Web use the vsepr model to predict the molecular geometry of (a) sf4, (b) if5. The main idea is that electrons don’t like to hang around near each other because they repel each other. Are all five possible electronic geometries represented in this set of molecules? Compounds 1 and 2 are both polar. There are lone pairs on x or other atoms, but we don't care. Hybridization and resonance structures identify: Determine the electron geometry and molecular geometry of the following molecules using the vsepr model. If not, which ones are missing? Web vsepr theory states that regions of high electron density, such as bonding pairs or lone pairs of electrons (a vsepr or electron domain), will arrange themselves as far apart as possible around the central atom. What is the main idea behind vsepr theory? Web lewis structures & vsepr theory worksheet name_____ chemical formula lewis structure central atom(s) list each central atom (a) total number of electron pairs on each central atom (used to determine base shape) number of bond pairs of electrons (x) number of electron lone pairs (e) vsepr geometries 1. Web sch4u1 molecular geometry and vsepr name: Bi3 ch4 nf3 c2h2 everett community college tutoring center student support services program Web 1) what is the main idea behind vsepr theory? Web vsepr worksheet 1) briefly describe the primary ideas behind vsepr theory. Compounds 1 and 2 are both nonpolar. Compound 1 is polar, whereas compound 2 is nonpolar. Web vsepr worksheet 318 briefly describe the primary ideas behind vsepr theory.vsepr theory worksheet answers
Vsepr Worksheet printable pdf download
Vsepr Worksheet With Answers
Electron Configuration Worksheet Answer Key Pogil / Matt Barry
Vsepr theory chart
Printable Vsepr Chart Printable Blank World
20 Vsepr Worksheet High School
Lewis Structure And Molecular Geometry Worksheet —
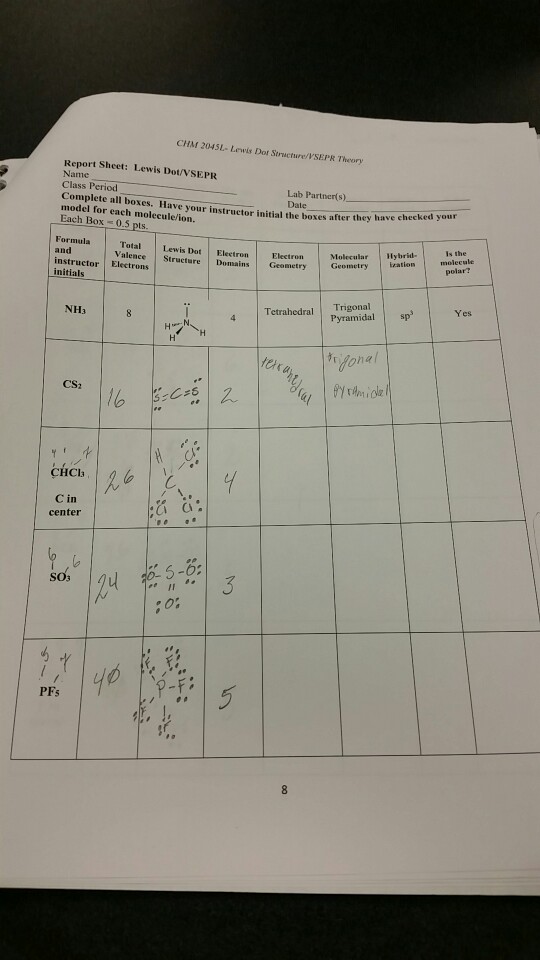
Solved CHM 2045L Lewis Dot Structure/VSEPR Theory Report...
Lewis Structure And Vsepr Worksheet
Compound 1 Is Nonpolar, Whereas Compound 2 Is Polar.
2) For Each Of The Following Compounds, A Lewis Structure, Determine The Bond Angles And Molecular Shapes For All Atoms:
Web The Vsepr (Valence Shell Electron Pair Repulsion) Model, Which States That Electron Pairs Around A Central Atoms Will Assume A Geometry That Keeps Them As Far Apart From Each Other As Possible.
How Does Molecule Shape Change With Different Numbers Of Bonds And Electron Pairs?
Related Post: