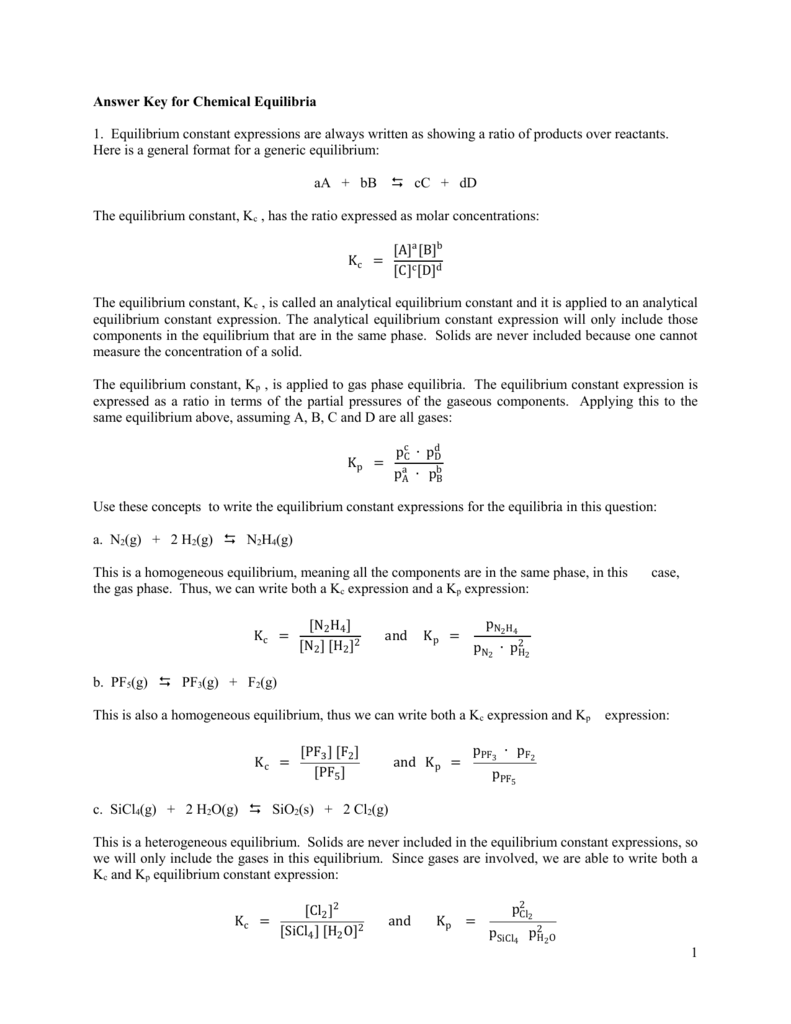
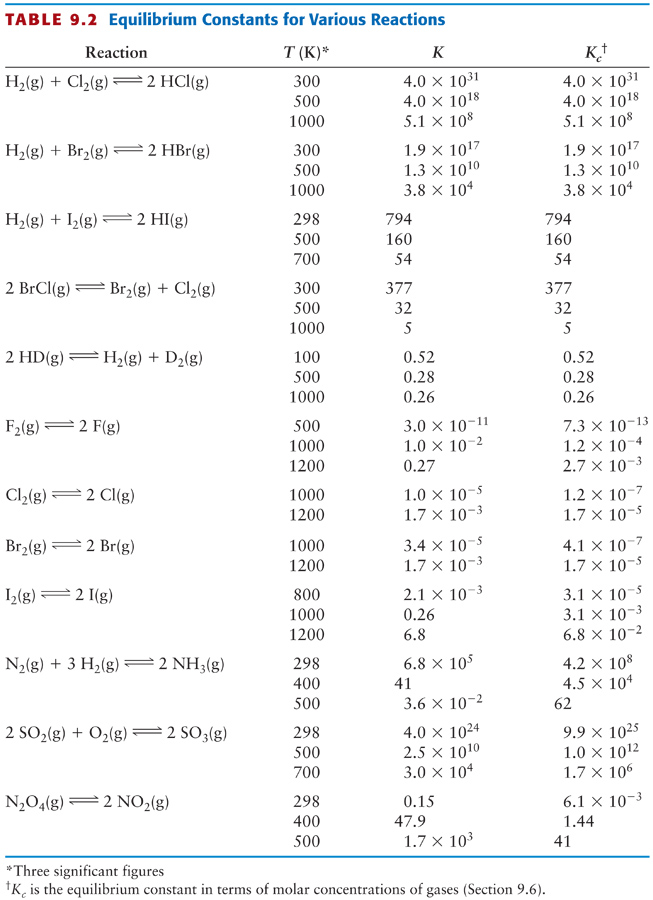
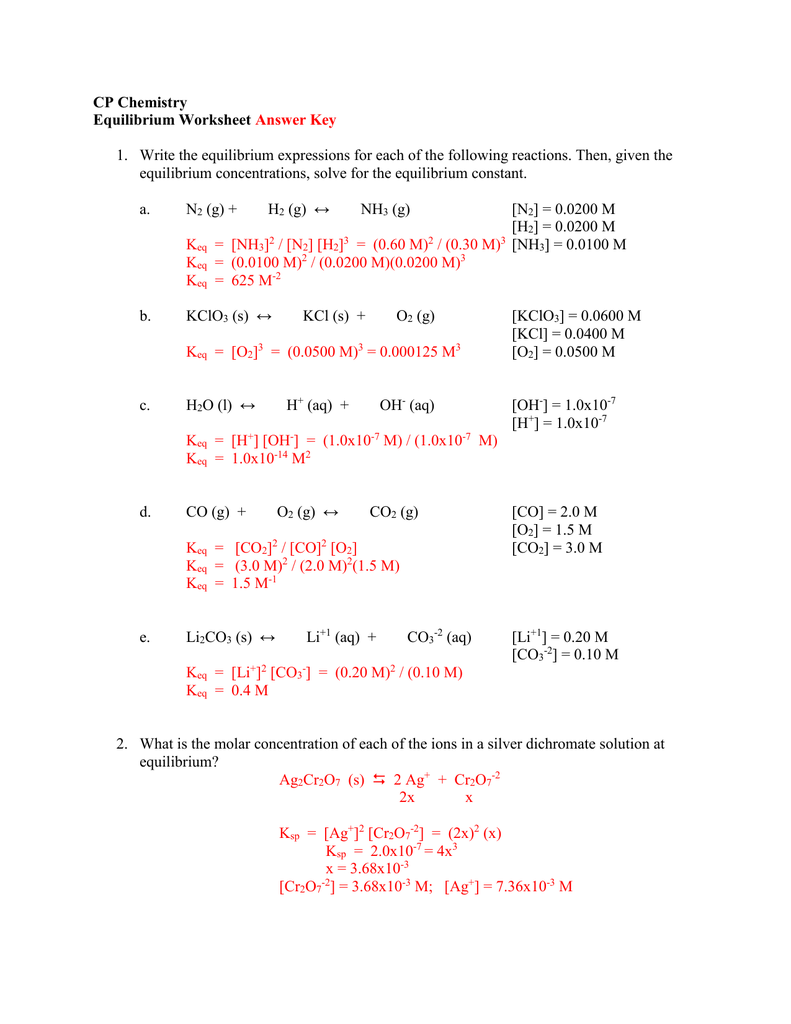
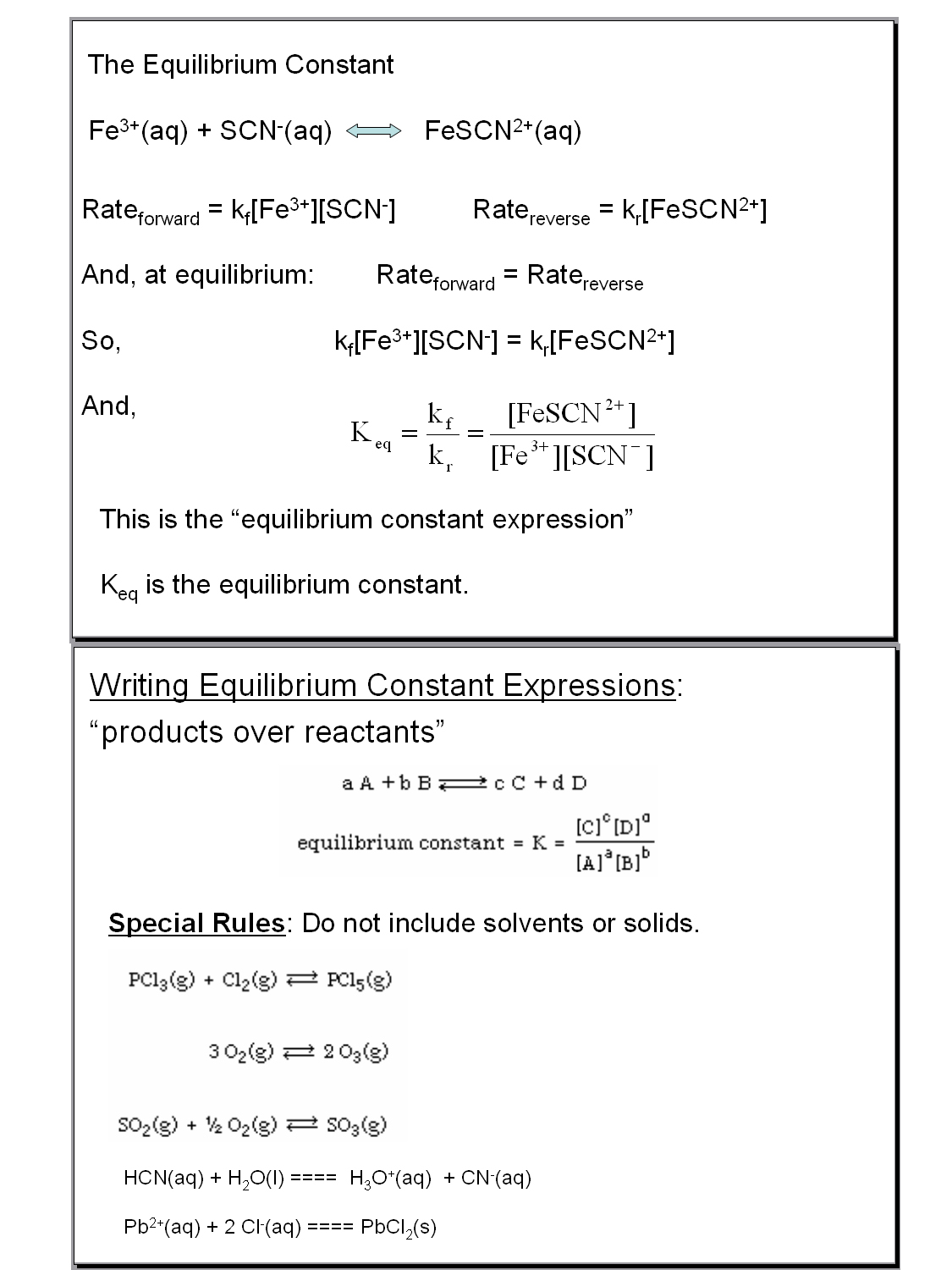
Worksheet 1 Equilibrium Constants Answer Key
Worksheet 1 Equilibrium Constants Answer Key - Web understand how to set up a calculation of all species concentrations at equilibrium, given initial concentrations and the value of the equilibrium constant. The rates of the forward and reverse reactions are equal. Does the position at equilibrium depend upon how the equilibrium was reached? Web up to 24% cash back equilibrium constant worksheet 1 1. Web this discussion worksheet addresses equilibria and equilibrium constants. Write the equilibrium expression for the following reactions: H2 (g) + i2 (g) 2 hi (g) kc = 50.5. [h2] = [i2] = 0.360mol / l, [hi] = 2.50mol / l. At equilibrium the concentration of. Web ch213 equilibrium constant at equilibrium, the ratio of the concentrations of products to reactants is constant. Web ch213 equilibrium constant at equilibrium, the ratio of the concentrations of products to reactants is constant. If the temperature of an exothermic reaction at equilibrium is lowered, is the. Web ch 102 discussion worksheet #7 summer 2023 1 answers to discussion worksheet 7 1. Web keq and le chatelier wks. [h2] = [i2] = 0.360mol / l, [hi] =. Web understand how to set up a calculation of all species concentrations at equilibrium, given initial concentrations and the value of the equilibrium constant. Q = [hi]2 [h2][i2] = (2.50mol / l)2 (0.360mol / l)2 = 48.2. W w + xx ⇌ uu + vv v = [u] u [v ] [w] w [x] x h 2 o (g). Web. Q = [hi]2 [h2][i2] = (2.50mol / l)2 (0.360mol / l)2 = 48.2. Web a) calculate the equilibrium concentrations of all species for the following reaction if the initial concentrations of h2 and i2 are both 1.00 m. Web ch213 equilibrium constant at equilibrium, the ratio of the concentrations of products to reactants is constant. W w + xx ⇌. Web the concentration of reactants and products are constant.(not equal) macroscopic properties are constant (color, mass, density, pressure, concentrations). Is this an endothermic or exothermic reaction (in the forward. [h2] = [i2] = 0.360mol / l, [hi] = 2.50mol / l. At equilibrium the concentration of. The keq for this reaction at 25℃ is 1.02. W w + xx ⇌ uu + vv v = [u] u [v ] [w] w [x] x h 2 o (g). Q = [hi]2 [h2][i2] = (2.50mol / l)2 (0.360mol / l)2 = 48.2. Write the equilibrium expression for the following reactions: A basic introduction to equilibrium constants should be given before hand. Web keq and le chatelier wks. Web the concentration of reactants and products are constant.(not equal) macroscopic properties are constant (color, mass, density, pressure, concentrations). Web understand how to set up a calculation of all species concentrations at equilibrium, given initial concentrations and the value of the equilibrium constant. Write the equilibrium expression for the following reactions: (a) i 2 is the catalyst; Is this an. Web calculate the equilibrium constant given the reaction below: At equilibrium the concentration of. W w + xx ⇌ uu + vv v = [u] u [v ] [w] w [x] x h 2 o (g). A) what is the equilibrium constant expression. The rates of the forward and reverse reactions are equal. Web the concentration of reactants and products are constant.(not equal) macroscopic properties are constant (color, mass, density, pressure, concentrations). The rates of the forward and reverse reactions are equal. H2 (g) + i2 (g) 2 hi (g) kc = 50.5. 2 (𝑔) +?2 (𝑔) k = 0.020 5. W w + xx ⇌ uu + vv v = [u] u. Write equilibrium constant expressions for the following reactions as written belowand for the reverse reaction (products become. 2 (𝑔) +?2 (𝑔) k = 0.020 5. At equilibrium the concentration of. Web understand how to set up a calculation of all species concentrations at equilibrium, given initial concentrations and the value of the equilibrium constant. Q = [hi]2 [h2][i2] = (2.50mol. The keq for this reaction at 25℃ is 1.02. Web up to 24% cash back equilibrium constant worksheet 1 1. The ratio value is called the equilibrium constant (in terms of. (b) step 1 is the slowest step since it depends on both ch 3cho. H2 (g) + i2 (g) 2 hi (g) kc = 50.5. The keq for this reaction at 25℃ is 1.02. W w + xx ⇌ uu + vv v = [u] u [v ] [w] w [x] x h 2 o (g). Write the equilibrium expression for the following reactions: At equilibrium the concentration of. Web the equilibrium constant, k, is used to determine the relative concentrations of products and reactants at equilibrium. According to the law of mass action, if a chemical reaction has. A basic introduction to equilibrium constants should be given before hand. Web ch 102 discussion worksheet #7 summer 2023 1 answers to discussion worksheet 7 1. Web calculate the equilibrium constant given the reaction below: Web at 573 k, the equilibrium constant for: 2 (𝑔) +?2 (𝑔) k = 0.020 5. The ratio value is called the equilibrium constant (in terms of. H2 (g) + i2 (g) 2 hi (g) kc = 50.5. Write equilibrium constant expressions for the following reactions as written belowand for the reverse reaction (products become. [h2] = [i2] = 0.360mol / l, [hi] = 2.50mol / l. Write the equilibrium expression for the following reactions: Does the position at equilibrium depend upon how the equilibrium was reached? Q = [hi]2 [h2][i2] = (2.50mol / l)2 (0.360mol / l)2 = 48.2. Web the concentration of reactants and products are constant.(not equal) macroscopic properties are constant (color, mass, density, pressure, concentrations). A) what is the equilibrium constant expression. Web up to 24% cash back equilibrium constant worksheet 1 1. Web at 573 k, the equilibrium constant for: Web a) calculate the equilibrium concentrations of all species for the following reaction if the initial concentrations of h2 and i2 are both 1.00 m. Q = [hi]2 [h2][i2] = (2.50mol / l)2 (0.360mol / l)2 = 48.2. Write the equilibrium expression for the following reactions: Since the q value is less than k (48.2<54.8), the system is. Web the equilibrium constant, k, is used to determine the relative concentrations of products and reactants at equilibrium. Web calculate the equilibrium constant given the reaction below: Web ch 102 discussion worksheet #7 summer 2023 1 answers to discussion worksheet 7 1. At equilibrium the concentration of. H2 (g) + i2 (g) 2 hi (g) kc = 50.5. The rates of the forward and reverse reactions are equal. Is this an endothermic or exothermic reaction (in the forward. If the temperature of an exothermic reaction at equilibrium is lowered, is the. Web calculations using the equilibrium constant name using the equilibrium constant expressions you determined on page 79, calculate the value of k when: Web 1) write all equilibrium equations 2) write all equilibrium concentrations 3) write all equilibrium expressions set a:Answer Key for Chemical Equilibria 1. Equilibrium constant
Equilibrium Expressions Worksheet worksheet
ANSWER Worksheet 1 Approaching Equilibrium. shalini ravi shalini
Equilibrium Constant Worksheet Worksheets For Kindergarten
CP Chemistry
Chapter 18.1 Waves Answer Key Islero Guide Answer for
Equilibrium And Pressure Gizmo Answer Key / Solved Activity B The
03 Equilibrium Constant Worksheet 2 Answers EQUILIBRIUM CONSTANT
️Worksheet Calculating Keq Free Download Gmbar.co
Class 10 Notes Chemistry Chemical Equilibrium Review Questions Notes
The Keq For This Reaction At 25℃ Is 1.02.
Write Equilibrium Constant Expressions For The Following Reactions As Written Belowand For The Reverse Reaction (Products Become.
Write The Equilibrium Expression For The Following Reactions:
Web Understand How To Set Up A Calculation Of All Species Concentrations At Equilibrium, Given Initial Concentrations And The Value Of The Equilibrium Constant.
Related Post: