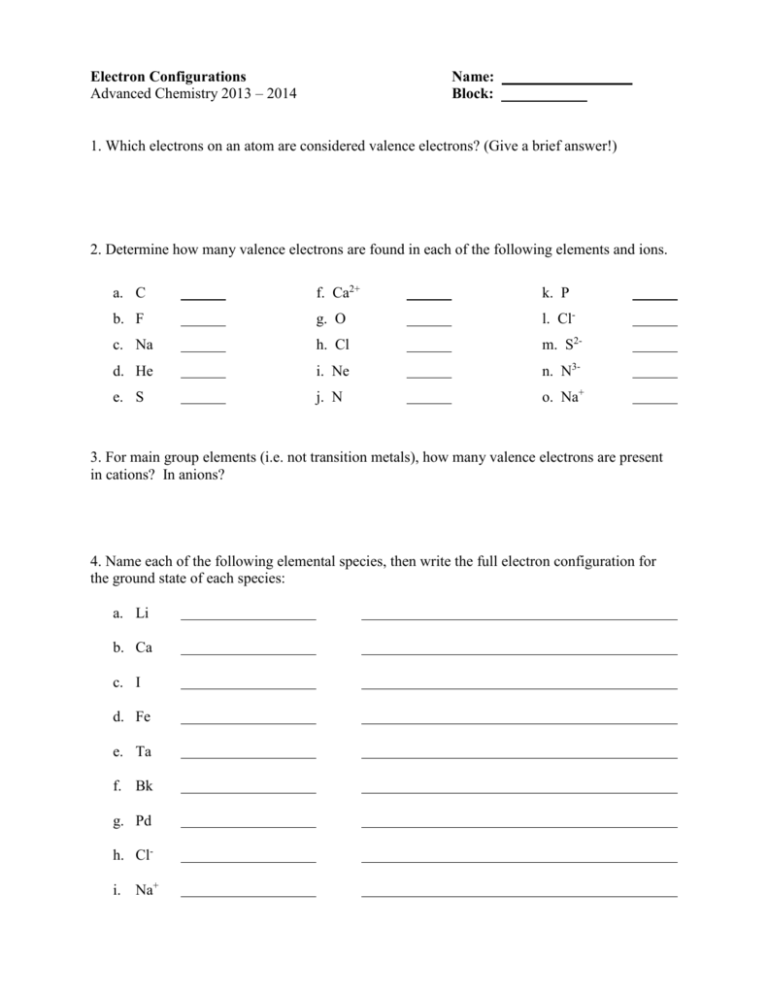
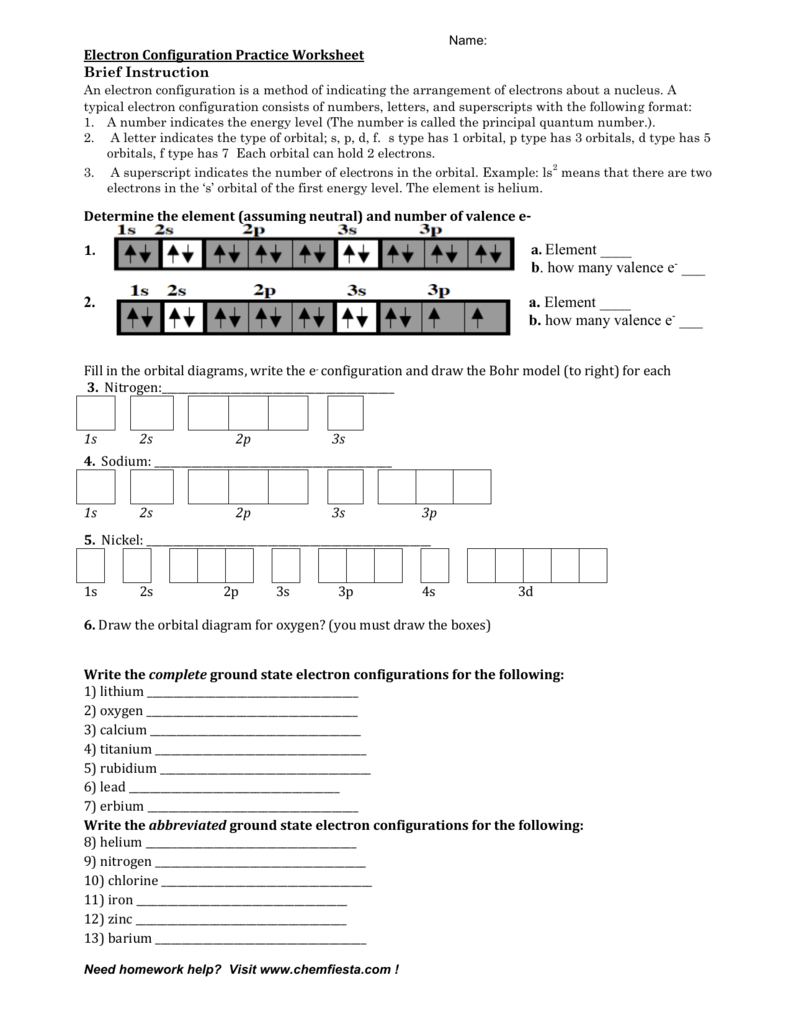
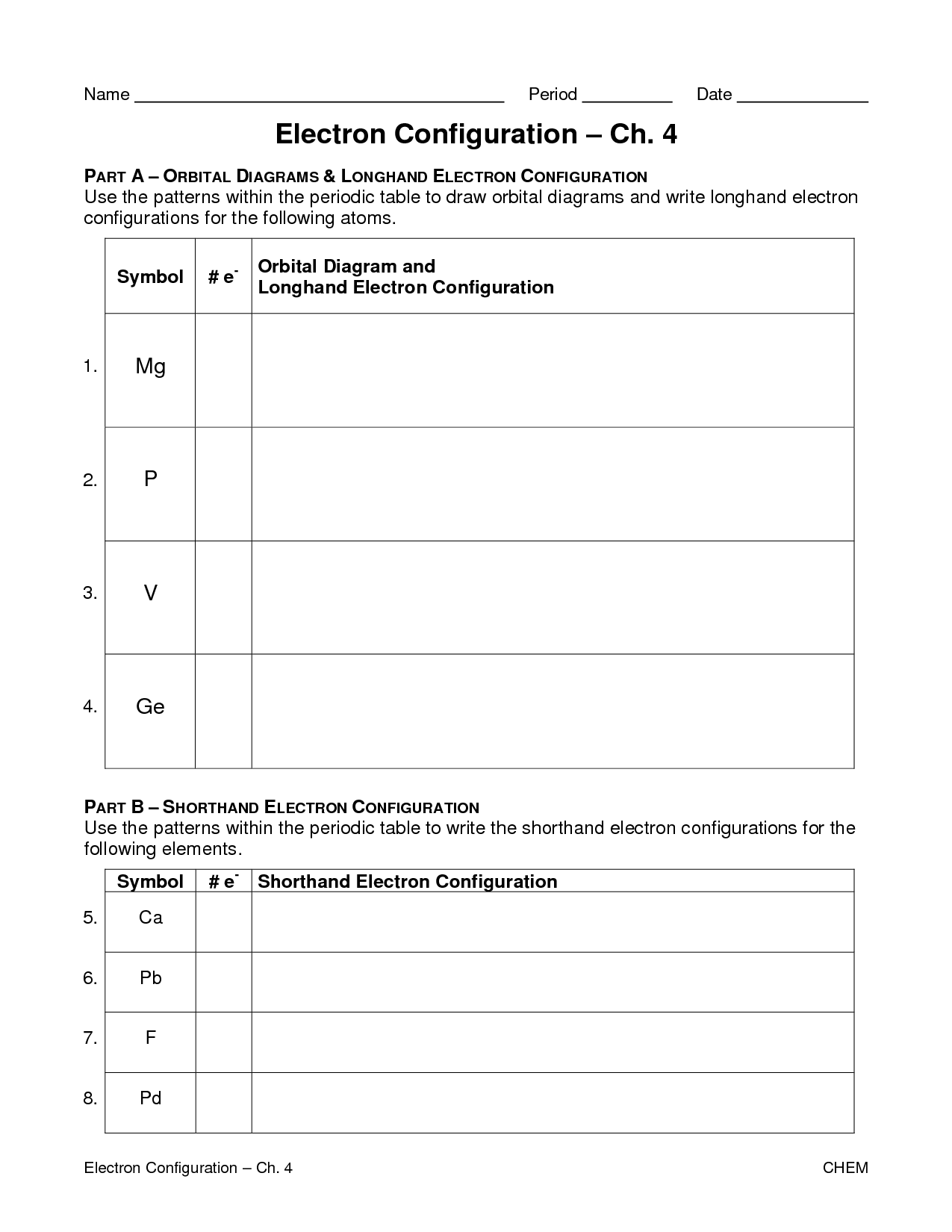
Worksheet Electron Configuration
Worksheet Electron Configuration - Periodic table using spdf spdf notation, what is the electron configuration for a neutral atom of beryllium? Oxygen (o) oxygen o is element 8. Some of the worksheets for this concept are electron configuration practice work, electron configuration work, electron configuration practice work, chemistry 143 electron configurations caddell, atomic structure and electron configurations multiple, exam 4 collected essays, 13. An answer scheme is provided to allow for peer marking. Web docx, 146.5 kb. The “ground state” electron configuration is the lowest energy combination of electrons in the atomic orbitals. Web electron configuration practice worksheet key. Web electron configuration worksheets with answers (extensive guide to solve) definition and basics of electronic configuration. This is sometimes called the , or the ‘solar system’, model. Web help your students learn how to write electron orbital diagrams and electron configurations with this long, detailed worksheet set!this worksheet set includes 6+ pages of problems for students to complete, including completed examples and a complete answer key (pictured above). The easiest and most reliable technique for writing electron configurations is to use the periodic table as your guide. 1 s^2 2 s^2 1s22s2 a 1 s^2 2 s^2 1s22s2 1 s^2 2 s^1 2p^1 1s22s12p1 b 1 s^2 2 s^1 2p^1 1s22s12p1 1 s^2 2 p^2 1s22p2 c 1 s^2 2 p^2 1s22p2 1 s^2 1 p^2 1s21p2 d. Web an electron configuration is a method of indicating the arrangement of electrons about a nucleus. Suitable for grade 8 upward. Web electron configuration practice worksheet on a separate piece on paper, write the electron configurations, orbital filling diagrams, and electron dot structures of the following elements: The easiest and most reliable technique for writing electron configurations is to use. 1 s^2 2 s^2 1s22s2 a 1 s^2 2 s^2 1s22s2 1 s^2 2 s^1 2p^1 1s22s12p1 b 1 s^2 2 s^1 2p^1 1s22s12p1 1 s^2 2 p^2 1s22p2 c 1 s^2 2 p^2 1s22p2 1 s^2 1 p^2 1s21p2 d Trends in the periodic table simplified. Web help your students learn how to write electron orbital diagrams and electron. Web this worksheet provides extra practice for writing electron configurations. The electron configurations in this worksheet assume that lanthanum (la) is the first element in the 4f block and that actinium (ac) is the first element in the 5f block. Some of the worksheets for this concept are 12 electron energy and light t, lewis electron dot structure answers, electron. The easiest and most reliable technique for writing electron configurations is to use the periodic table as your guide. Web help your students learn how to write electron orbital diagrams and electron configurations with this long, detailed worksheet set!this worksheet set includes 6+ pages of problems for students to complete, including completed examples and a complete answer key (pictured above).. Students will answer questions about energy levels, sublevels, and orbitals. Web an electron configuration is a method of indicating the arrangement of electrons about a nucleus. An answer scheme is provided to allow for peer marking. A letter indicates the type of orbital; Designed for btec level 2 applied science atomic structure assignment. [he] 2s2 nitrogen orbital notation: Students will answer questions about energy levels, sublevels, and orbitals. Web help your students learn how to write electron orbital diagrams and electron configurations with this long, detailed worksheet set!this worksheet set includes 6+ pages of problems for students to complete, including completed examples and a complete answer key (pictured above). Suitable for grade 8. The “ground state” electron configuration is the lowest energy combination of electrons in the atomic orbitals. Designed for btec level 2 applied science atomic structure assignment. Web an electron configuration is a method of indicating the arrangement of electrons about a nucleus. Sodium iron bromine barium thallium cobalt silver tellurium radium astatine lithium oxygen calcium titanium rubidium The electron configurations. Some of the worksheets for this concept are electron configuration practice work, electron configuration work, electron configuration practice work, chemistry 143 electron configurations caddell, atomic structure and electron configurations multiple, exam 4 collected essays, 13. This is sometimes called the , or the ‘solar system’, model. Web this worksheet from teach simple tests students’ knowledge of standard and noble gas. Add to my workbooks (65) 1 explains the atom and how electrons are arranged, the next requires students to draw electron configuration diagrams for the first 20 elements. Web since there are 2 electrons in the box labeled 1s, 2 electrons in the box labeled 2s, and also 2 electrons in the boxes labeled 2p we say the c electron. As we all already know, electrons bear charge i.e. Web electron configuration practice worksheet key. Copper 1s22s22p63s23p64s23d9 2) iodine 1s22s22p63s23p64s23d104p65s24d105p5 potassium 1s22s22p63s23p64s1 bismuth 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p3 zirconium 1s22s22p63s23p64s23d104p65s24d2 The easiest and most reliable technique for writing electron configurations is to use the periodic table as your guide. The electron configurations in this worksheet assume that lanthanum (la) is the first element in the 4f block and that actinium (ac) is the first element in the 5f block. The electron configurations in this worksheet assume that lanthanum (la) is the first element in the 4f block and that actinium (ac) is the first element in the 5f block. Web since there are 2 electrons in the box labeled 1s, 2 electrons in the box labeled 2s, and also 2 electrons in the boxes labeled 2p we say the c electron configuration in orbital notation is 1s 2 2s 2 2p 2. Periodic table using spdf spdf notation, what is the electron configuration for a neutral atom of beryllium? Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. 1 explains the atom and how electrons are arranged, the next requires students to draw electron configuration diagrams for the first 20 elements. Sodium iron bromine barium thallium cobalt silver tellurium radium astatine lithium oxygen calcium titanium rubidium The electron configuration of an element is a standard representation. Some of the worksheets for this concept are 12 electron energy and light t, lewis electron dot structure answers, electron configuration work name vandenboutlabrake, electron configuration work, work 12, electron configuration practice work, atomic structure and electron configurations. Oxygen (o) oxygen o is element 8. Web the electron configuration is a listing of which atomic orbitals are occupied by electrons, and how many electrons are in each type of atomic orbital. Web an electron configuration is a method of indicating the arrangement of electrons about a nucleus. Suitable for grade 8 upward. In the space below, write the unabbreviated electron configurations of the following elements: Web write the unabbreviated electron configurations of the following elements: Detailed explanation of atomic orbitals. Web this worksheet provides extra practice for writing electron configurations. Students will answer questions about energy levels, sublevels, and orbitals. Designed for btec level 2 applied science atomic structure assignment. Web an electron configuration is a method of indicating the arrangement of electrons about a nucleus. A number indicates the energy level (the number is called the principal quantum number.). This is sometimes called the , or the ‘solar system’, model. The electron configurations in this worksheet assume that lanthanum (la) is the first element in the 4f block and that actinium (ac) is the first element in the 5f block. Sodium iron bromine barium thallium cobalt silver tellurium radium astatine lithium oxygen calcium titanium rubidium Some of the worksheets for this concept are 12 electron energy and light t, lewis electron dot structure answers, electron configuration work name vandenboutlabrake, electron configuration work, work 12, electron configuration practice work, atomic structure and electron configurations. An answer scheme is provided to allow for peer marking. In the space below, write the unabbreviated electron configurations of the following elements: 1 explains the atom and how electrons are arranged, the next requires students to draw electron configuration diagrams for the first 20 elements. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Detailed explanation of atomic orbitals. The electron configurations in this worksheet assume that lanthanum (la) is the first element in the 4f block and that actinium (ac) is the first element in the 5f block. Web electron configuration elements (atoms) and ions.Valence Electrons Worksheet Key
Electron Configuration Practice Worksheet Education Template
Electron Configuration worksheet
Electron Configurations Pacticew Worksheet With Key / E L E C T R O N C
Electron Configuration Practice Worksheet
Electron Configurations Pacticew Worksheet With Key Electron
Chemistry Electron Configurations Worksheet
Exercise Electron Configurations Worksheet Electron Configurations
Electron Configuration Practice Worksheet
13 Best Images of Electron Configuration Worksheet With Answers
2 Worksheets On Electron Configuration.
[He] 2S2 Nitrogen Orbital Notation:
Web Since There Are 2 Electrons In The Box Labeled 1S, 2 Electrons In The Box Labeled 2S, And Also 2 Electrons In The Boxes Labeled 2P We Say The C Electron Configuration In Orbital Notation Is 1S 2 2S 2 2P 2.
Suitable For Grade 8 Upward.
Related Post: