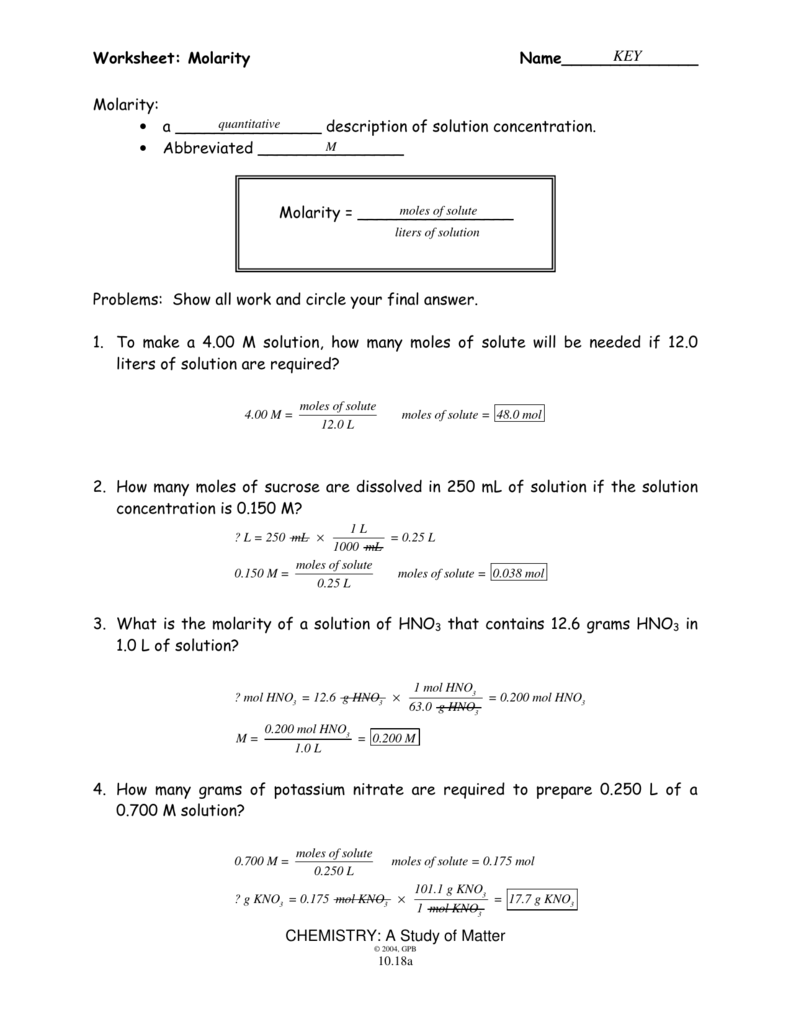
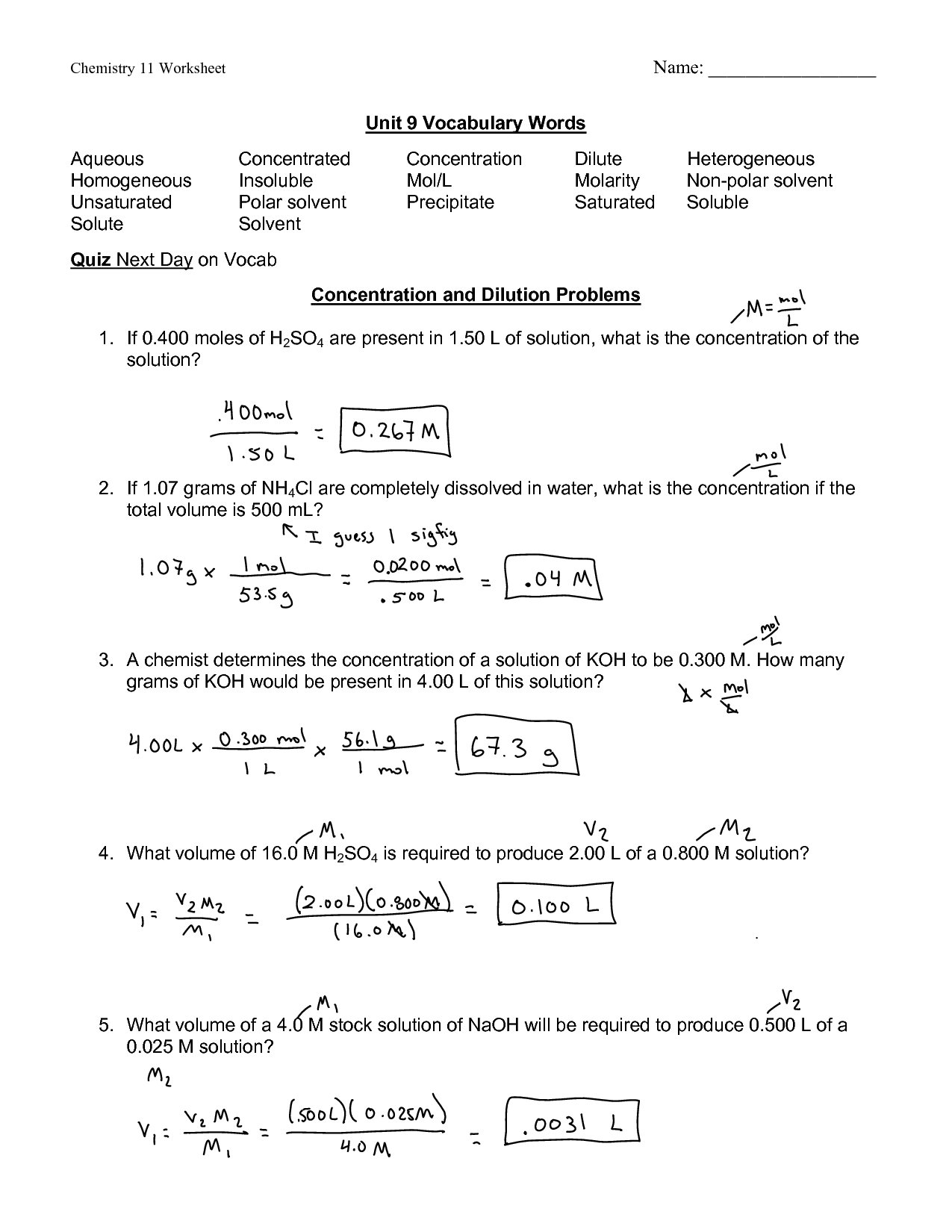
Worksheet Molarity Answers
Worksheet Molarity Answers - When the solvent is water, we have an aqueous solution. Learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. Web molarity practice problems #1 grams of potassium carbonate are needed to make 280 ml of a 2.5 m solution? Another way to specify an amount is percentage composition by mass (or mass percentage, %. Web molarity = _____ problems: Web when using molarity to measure concentration you must follow the formula below and then put a capital m at the end of your answer to let the world know you used the molarity. How many liters of water are needed to make a 4.00 m solution. Web molarity worksheet answers return to solutions m enu return to worksheet go to m olarity the equations i will use are: Web molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. Molarity = moles of solute / volume of solution in litre. Molarity is represented by the following equation: Web mathematical manipulation of molality is the same as with molarity. Web molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. How many liters of water are needed to make a 4.00 m solution. Learn about the relationships between moles, liters,. Show all work and circle your final answer. Web molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. Web molarity worksheet answers return to solutions m enu return to worksheet go to m olarity the equations i will use are: Molarity = moles of solute / volume of. When the solvent is water, we have an aqueous solution. Description simple worksheet used for middle school lesson on concentration of solutions: Another way to specify an amount is percentage composition by mass (or mass percentage, %. Web molarity practice problems #1 grams of potassium carbonate are needed to make 280 ml of a 2.5 m solution? Web molarity extra. Description simple worksheet used for middle school lesson on concentration of solutions: Web mathematical manipulation of molality is the same as with molarity. What is the molarity of 500. Web molarity problems worksheet use m or mol/l as unit for molarity. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Description simple worksheet used for middle school lesson on concentration of solutions: = moles of solute / liters of solution and v = grams /. Web molarity extra practice worksheet 1. 1) in this problem, simply solve using the molarity equation to find that. We already have the molarity and volume of solution, both of. Web mathematical manipulation of molality is the same as with molarity. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Do not confuse m, l, and ml! = moles of solute / liters of solution and v = grams /. When the solvent is water, we have an aqueous solution. Show all work and circle your final answer. We already have the molarity and volume of solution, both of. Web when using molarity to measure concentration you must follow the formula below and then put a capital m at the end of your answer to let the world know you used the molarity. When the solvent is water, we have. Web molarity practice problems #1 grams of potassium carbonate are needed to make 280 ml of a 2.5 m solution? To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and water? Web the molarity. Web molarity worksheet w 331 everett community college student support services program what is the molarity of the following solutions given that: Web molarity worksheet answers return to solutions m enu return to worksheet go to m olarity the equations i will use are: What is the molarity of 2.0 l of solution made from 2.4 moles of nacl and. Molarity = moles of solute / volume of solution in litre. = moles of solute / liters of solution and v = grams /. Web the molarity of a solution is defined as the moles of solute per liter of solution. We already have the molarity and volume of solution, both of. Remember that 1 liter = 1000 ml. Web when using molarity to measure concentration you must follow the formula below and then put a capital m at the end of your answer to let the world know you used the molarity. What is the molarity of 500. Description simple worksheet used for middle school lesson on concentration of solutions: Learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. Web the molarity of a solution is defined as the moles of solute per liter of solution. Another way to specify an amount is percentage composition by mass (or mass percentage, %. Web molarity extra practice worksheet 1. Web molarity problems worksheet use m or mol/l as unit for molarity. Web what determines the concentration of a solution? Molarity = moles of solute / volume of solution in litre. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. Web molarity worksheet w 331 everett community college student support services program what is the molarity of the following solutions given that: Web molarity = _____ problems: Do not confuse m, l, and ml! To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. Web molarity worksheet answers return to solutions m enu return to worksheet go to m olarity the equations i will use are: 1) in this problem, simply solve using the molarity equation to find that. Web molarity practice problems #1 grams of potassium carbonate are needed to make 280 ml of a 2.5 m solution? = moles of solute / liters of solution and v = grams /. Web molarity = _____ problems: To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. Web molarity extra practice worksheet 1. Description simple worksheet used for middle school lesson on concentration of solutions: = moles of solute / liters of solution and v = grams /. Web molarity of a solution is equal to the number of moles of solute divided by the number of liters of solution. Web molarity practice problems #1 grams of potassium carbonate are needed to make 280 ml of a 2.5 m solution? Web molarity worksheet w 331 everett community college student support services program what is the molarity of the following solutions given that: What is the molarity of 500. Show all work and circle your final answer. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. Remember that 1 liter = 1000 ml. How many liters of water are needed to make a 4.00 m solution. Another way to specify an amount is percentage composition by mass (or mass percentage, %. We already have the molarity and volume of solution, both of. Show all work and circle your final answer. Molarity is represented by the following equation:️Stoichiometry Using Molarity Worksheet Answers Free Download Gambr.co
Molarity Worksheet 1 worksheet
Molarity Worksheet Answer Key
Molarity Worksheet Answer Key arainspire
Molarity Practice Worksheet Answer
Molarity, Molality, Normality, and Mass Percent Worksheet II Answer Key
Molarity Worksheet Answers Chemistry Promotiontablecovers
Molarity Worksheet Answers Chemistry Ivuyteq
7 Molarity Worksheet With Answers /
molarity worksheet with answers
Web Molarity = _____ Problems:
Web When Using Molarity To Measure Concentration You Must Follow The Formula Below And Then Put A Capital M At The End Of Your Answer To Let The World Know You Used The Molarity.
Web Molarity Worksheet Answers Return To Solutions M Enu Return To Worksheet Go To M Olarity The Equations I Will Use Are:
Web Molarity = _____ Problems:
Related Post: