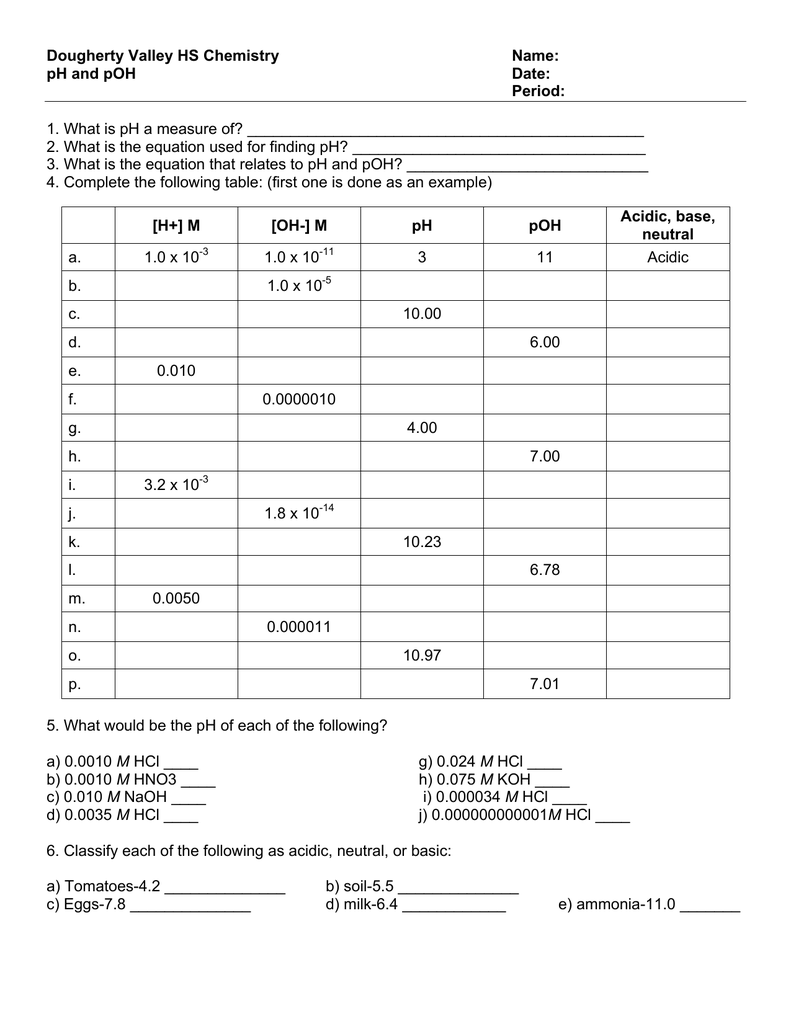
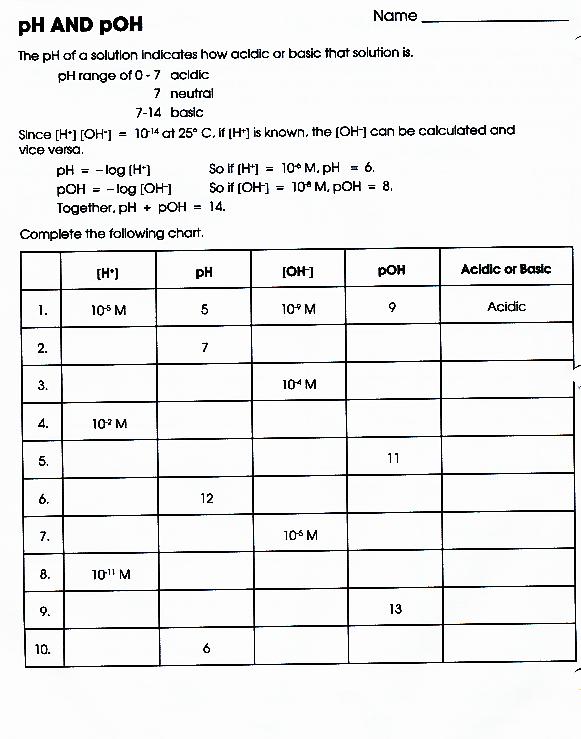
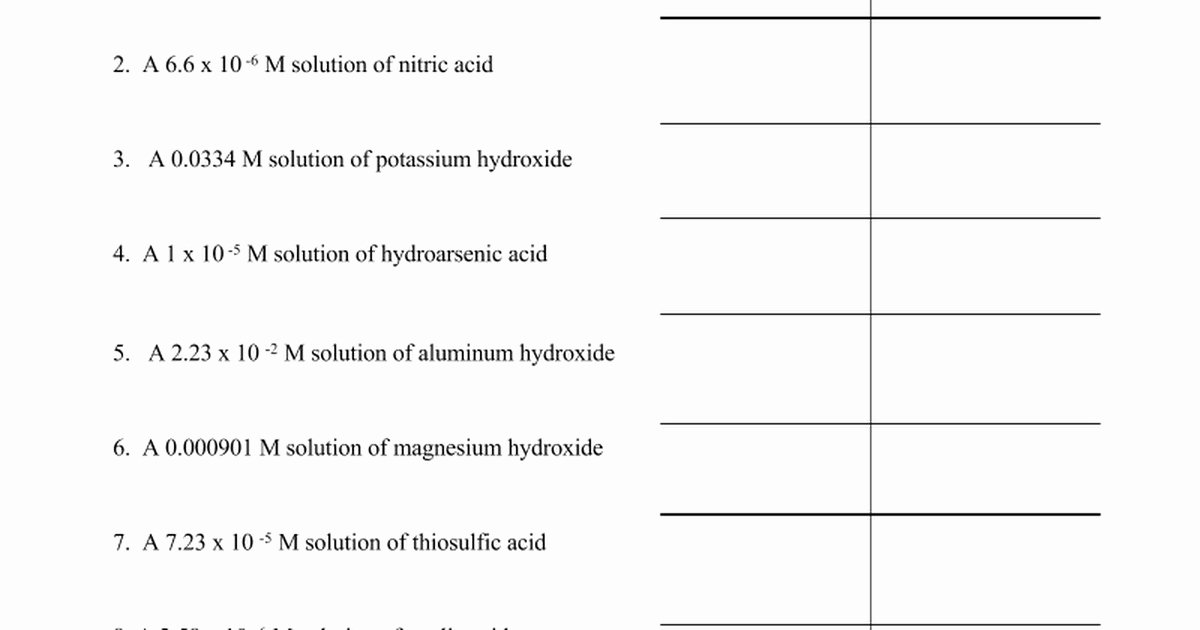
Worksheet Ph Calculations Answers
Worksheet Ph Calculations Answers - Web what is the ph of a solution of 0.36 m hcl, 0.62 m naoh, and 0.15 m hno 3 ? Solution of a weak acid or mixture of a. 3.00 2) a 0.09 m solution of hbr (hydrobromic acid). Web up to 6% cash back the question asks us to find the ph of the solution, so we will need to convert poh to ph. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Hydrochloric acid and nitric acid are strong acids, and sodium hydroxide is a strong base; Worked answers hcl is a strong acid, so [h+] =. The ph of the solution is: The ph of the solution is. Web solution from equation 15.8.3, ph + poh = 14.00. The ph of the solution is. Worksheets are calculating ph and poh work, work ph calculations name,. Web a ph value of _____ indicates a neutral solution. To do so, we simply subtract the poh from 14. A ph value of more than 7 indicates a(n) _____ solution. A ph value of more than 7 indicates a(n) _____ solution. 3.00 2) a 0.09 m solution of hbr (hydrobromic acid). 3.00 2) a 0.09 m solution of hbr (hydrobromic acid). Worksheets are calculating ph and poh work, work ph calculations name,. Web in this video, we'll solve for [h₃o⁺] and ph in two different worked examples. 3.00 2) a 0.09 m solution of hbr (hydrobromic acid). The hydrogen concentration can be calculated by rearranging the equation for ph. Worksheets are calculating ph and poh work, work ph calculations name,. Web what is the ph of a solution of 0.36 m hcl, 0.62 m naoh, and 0.15 m hno 3 ? Hydrochloric acid and nitric acid are. 3.00 2) a 0.09 m solution of hbr (hydrobromic acid). First, we'll walk through the possible approaches for calculating [h₃o⁺] from poh. Web in this video, we'll solve for [h₃o⁺] and ph in two different worked examples. 3.00 2) a 0.09 m solution of hbr (hydrobromic acid). Autoionization of water and ph arrange the following solutions in the order of. Uopnps puy noá dn0jb jnoá ssnosya Autoionization of water and ph arrange the following solutions in the order of increasing acidity (least acidic to most acidic): From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11) = −. Solution of a weak acid or mixture of a. The ph of the solution is: First, we'll walk through the possible approaches for calculating [h₃o⁺] from poh. (a) ph = 9.8 (b) ph = 1.2 (c) ph = 4.7 (d) ph = 6.4. Web solution from equation 15.8.3, ph + poh = 14.00. Solution of a weak acid or mixture of a. Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! Web up to 6% cash back the question asks us to find the ph of the solution, so we will need to convert poh to ph. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! The ph of the solution is: Identify the type of solution resulting from the reaction. A ph value of more than 7. Solution of a weak acid or mixture of a. A ph value of more than 7 indicates a(n) _____ solution. Web solution from equation 15.8.3, ph + poh = 14.00. Web what is the ph of a solution of 0.36 m hcl, 0.62 m naoh, and 0.15 m hno 3 ? Identify the type of solution resulting from the reaction. From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11) = −. Web a ph value of _____ indicates a neutral solution. Identify the type of solution resulting from the reaction. Web instructions encourage learners to make a sensible estimate of the answer before attempting the calculation. Hydrochloric acid and nitric acid are strong acids, and sodium. From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11) = −. Solution of a weak acid or mixture of a. Worked answers hcl is a strong acid, so [h+] =. Autoionization of water and ph arrange the following solutions in the order of increasing acidity (least acidic to most acidic): Identify the type of solution resulting. Autoionization of water and ph arrange the following solutions in the order of increasing acidity (least acidic to most acidic): The hydrogen concentration can be calculated by rearranging the equation for ph. The ph of the solution is: First, we'll walk through the possible approaches for calculating [h₃o⁺] from poh. Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! Uopnps puy noá dn0jb jnoá ssnosya To do so, we simply subtract the poh from 14. Worked answers hcl is a strong acid, so [h+] =. From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11) = −. Identify the type of solution resulting from the reaction. Solution of a weak acid or mixture of a. (a) ph = 9.8 (b) ph = 1.2 (c) ph = 4.7 (d) ph = 6.4. The ph of the solution is. Web solution from equation 15.8.3, ph + poh = 14.00. Hydrochloric acid and nitric acid are strong acids, and sodium hydroxide is a strong base; A ph value of more than 7 indicates a(n) _____ solution. Worksheets are calculating ph and poh work, work ph calculations name,. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Web what is the ph of a solution of 0.36 m hcl, 0.62 m naoh, and 0.15 m hno 3 ? 3.00 2) a 0.09 m solution of hbr (hydrobromic acid). Solution of a weak acid or mixture of a. 3.00 2) a 0.09 m solution of hbr (hydrobromic acid). Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! Web in this video, we'll solve for [h₃o⁺] and ph in two different worked examples. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Show all work and circle the final answer. (a) ph = 9.8 (b) ph = 1.2 (c) ph = 4.7 (d) ph = 6.4. Web solution from equation 15.8.3, ph + poh = 14.00. First, we'll walk through the possible approaches for calculating [h₃o⁺] from poh. Autoionization of water and ph arrange the following solutions in the order of increasing acidity (least acidic to most acidic): The ph of the solution is: Identify the type of solution resulting from the reaction. Web what is the ph of a solution of 0.36 m hcl, 0.62 m naoh, and 0.15 m hno 3 ? Uopnps puy noá dn0jb jnoá ssnosya Hydrochloric acid and nitric acid are strong acids, and sodium hydroxide is a strong base; From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11) = −.Ph Worksheet Answer Key
Calculating Ph And Poh Worksheet
31 Ph Worksheet Answer Key Education Template
Ph Worksheet Answers Daily Pass
Acids And Bases Worksheet Ph GoodScienceWorksheets's Shop Teaching
Worksheet Ph Calculations Answers
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
Ph And Poh Worksheet Answers
ph and poh practice worksheet
Ph And Poh Worksheet Answers
To Do So, We Simply Subtract The Poh From 14.
Worked Answers Hcl Is A Strong Acid, So [H+] =.
Web Instructions Encourage Learners To Make A Sensible Estimate Of The Answer Before Attempting The Calculation.
Web A Ph Value Of _____ Indicates A Neutral Solution.
Related Post: