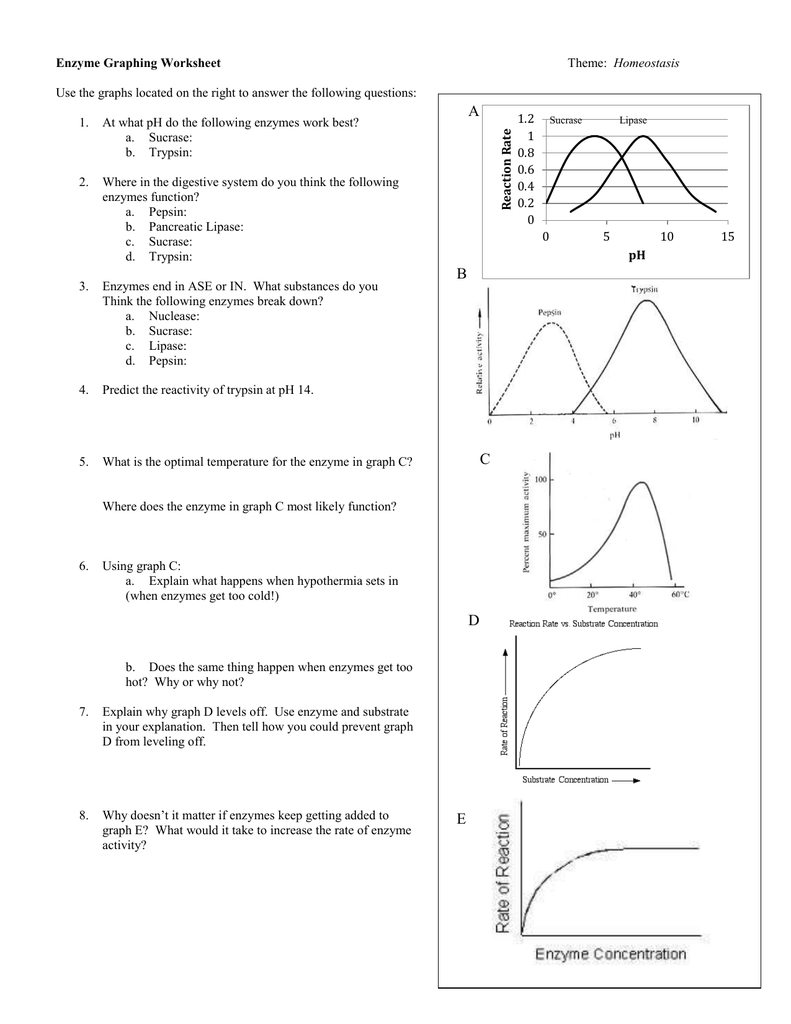
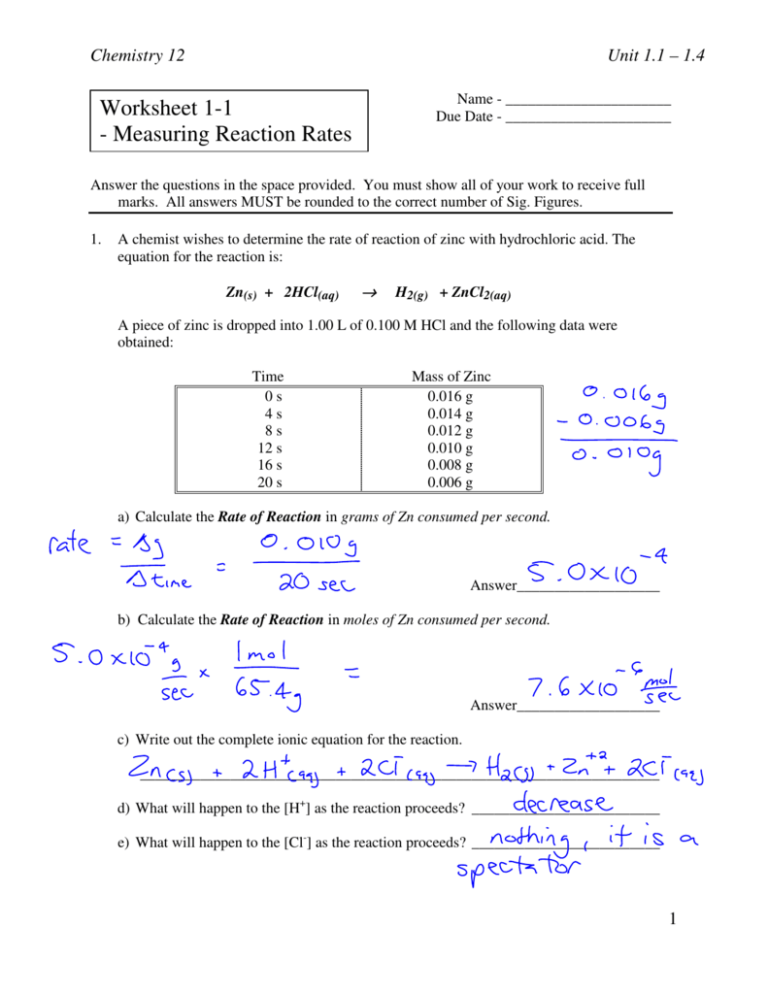
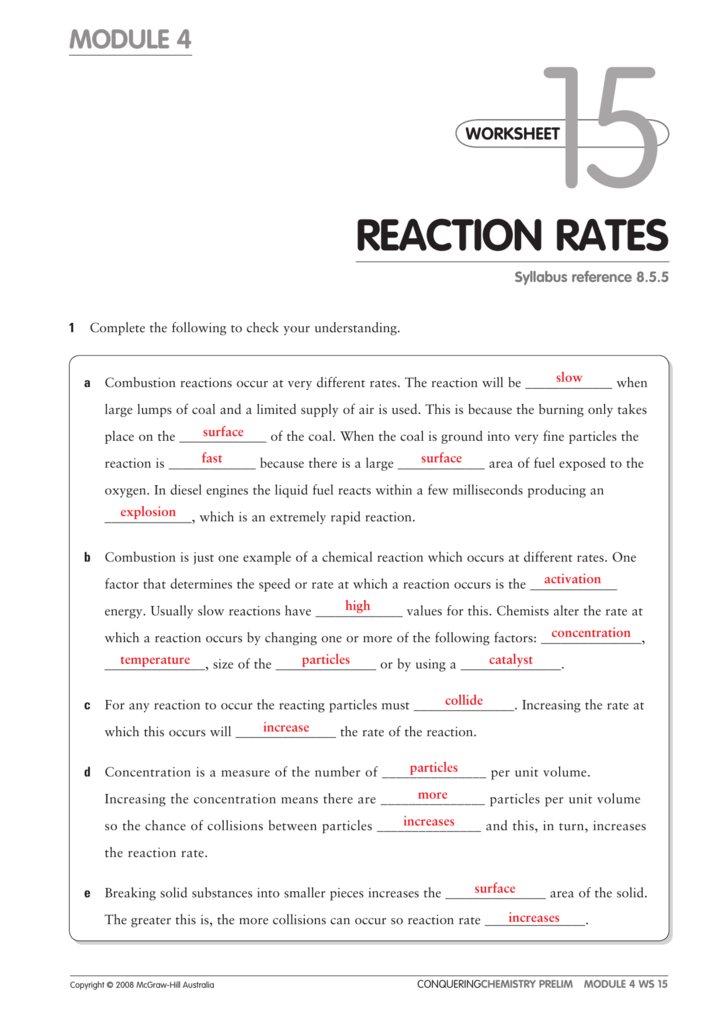
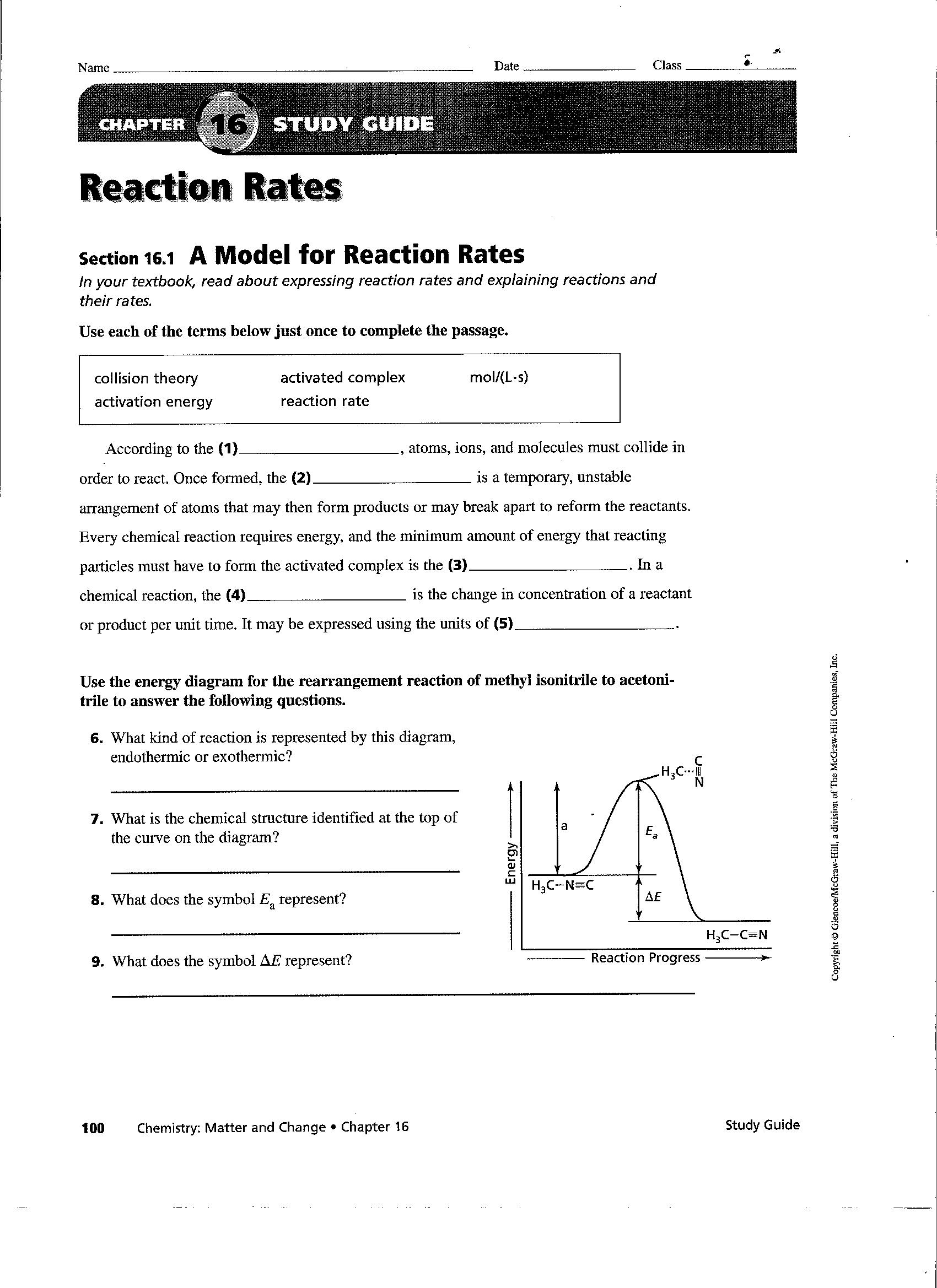
Worksheet Reaction Rates Answers
Worksheet Reaction Rates Answers - According to the collision theory, what 3. Some of the worksheets for this concept are potential energy diagram work answers, chemistry. Calculate the mean rate of reaction from 0 to 30 seconds. B) the average rate of reaction over the first 60 seconds. What affects the rate of a reaction? Web calculating rates of reaction: Fill the beaker or cup with water up to the top of the masking tape. (s) + 6 o2 (g) → 6 h2o (g) +. Yes, since the rate law is written for the slowest step of the reaction,. Web a series of free high school chemistry video lessons. (a) rate = k[n 2o][o], (b) rate = k[o 2]2, (c) rate = k[clco][cl 2] 2. Fill the beaker or cup with water up to the top of the masking tape. The reaction between \ce {mg} (s) mg(s) and \ce {hcl} (aq) hcl(aq) is represented by the equation above. What affects the rate of a reaction? C) the instantaneous rate. Some of the worksheets for this concept are potential energy diagram work answers, chemistry. Yes, since the rate law is written for the slowest step of the reaction,. H2o + co2 as the reaction proceeds ? For the product being produced ? Web a series of free high school chemistry video lessons. Web answers to discussion worksheet 6 1. Web calculating rates of reaction: R=0.03 m/s for the product being produced. The reaction between \ce {mg} (s) mg(s) and \ce {hcl} (aq) hcl(aq) is represented by the equation above. Yes, since the rate law is written for the slowest step of the reaction,. The reaction between \ce {mg} (s) mg(s) and \ce {hcl} (aq) hcl(aq) is represented by the equation above. (a) rate = k[n 2o][o], (b) rate = k[o 2]2, (c) rate = k[clco][cl 2] 2. For the product being produced? H2o + co2 as the reaction proceeds ? C) the instantaneous rate of. Fill the beaker or cup with water up to the top of the masking tape. For the product being produced ? What happens to the concentrations of: Choose an answer and hit 'next'. Web explore what makes a reaction happen by colliding atoms and molecules. What happens to the concentrations of: C6h12o6 & o2 as the reaction proceeds ? For the product being produced ? B) the average rate of reaction over the first 60 seconds. Web a series of free high school chemistry video lessons. Yes, since the rate law is written for the slowest step of the reaction,. (s) + 6 o2 (g) → 6 h2o (g) +. Web answers to discussion worksheet 6 1. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the. C) the instantaneous rate of. For the product being produced? H2o + co2 as the reaction proceeds ? In a kinetics experiment, a \pu {0.080 g} 0.080 g. For the product being produced ? (s) + 6 o2 (g) → 6 h2o (g) +. Fill the beaker or cup with water up to the top of the masking tape. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the. What affects the rate of a reaction? According to the collision theory, what 3. B) the average rate of reaction over the first 60. (a) rate = k[n 2o][o], (b) rate = k[o 2]2, (c) rate = k[clco][cl 2] 2. Some of the worksheets for this concept are potential energy diagram work answers, chemistry. Web a series of free high school chemistry video lessons. B) the average rate of reaction over the first 60 seconds. 19 mean rate of reaction = quantity of reactant. Calculate the mean rate of reaction from 0 to 30 seconds. Choose an answer and hit 'next'. In a kinetics experiment, a \pu {0.080 g} 0.080 g. Some of the worksheets for this concept are potential energy diagram work answers, chemistry. Yes, since the rate law is written for the slowest step of the reaction,. Web b) the average rate of reaction over the first 60 seconds. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the. According to the collision theory, what 3. Fill the beaker or cup with water up to the top of the masking tape. Web calculating rates of reaction: C6h12o6 & o2 as the reaction proceeds ? For the product being produced ? Web explore what makes a reaction happen by colliding atoms and molecules. R=0.03 m/s for the product being produced. (s) + 6 o2 (g) → 6 h2o (g) +. (a) rate = k[n 2o][o], (b) rate = k[o 2]2, (c) rate = k[clco][cl 2] 2. Web answers to discussion worksheet 6 1. 19 mean rate of reaction = quantity of reactant used time taken mean. H2o + co2 as the reaction proceeds ? Web 2 worksheets consisting of 30 questions and answers related to calculating average rate of reactions, instantaneous rate of reactions, determining factors affecting. Fill the beaker or cup with water up to the top of the masking tape. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the. Yes, since the rate law is written for the slowest step of the reaction,. C) the instantaneous rate of. What happens to the concentrations of: Calculate the mean rate of reaction from 0 to 30 seconds. For the product being produced ? B) the average rate of reaction over the first 60 seconds. Web calculating rates of reaction: 19 mean rate of reaction = quantity of reactant used time taken mean. Web 2 worksheets consisting of 30 questions and answers related to calculating average rate of reactions, instantaneous rate of reactions, determining factors affecting. H2o + co2 as the reaction proceeds ? Web answers to discussion worksheet 6 1. C6h12o6 & o2 as the reaction proceeds ? Web b) the average rate of reaction over the first 60 seconds. What affects the rate of a reaction?️Worksheet Reaction Rates Answer Key Free Download Goodimg.co
WARNER'S SCIENCE WEBSITE SCH4USEM22020
Enzyme Reactions Worksheet Answer Key Word Worksheet
Reaction Order And Rate Law Expression Worksheet Answer Key Law Worksheet
Worksheet 11 Measuring Reaction Rates
Rates Of Reaction Worksheet Ivuyteq
Worksheet Reaction Rates Answers Exam Academy
13 Worksheet Reaction Rates Answer /
13 Worksheet Reaction Rates Answer /
Reaction Rates And Equilibrium Worksheets
Web Up To 24% Cash Back A) The Average Rate Of Reaction Over The First 10 Seconds.
The Reaction Between \Ce {Mg} (S) Mg(S) And \Ce {Hcl} (Aq) Hcl(Aq) Is Represented By The Equation Above.
For The Product Being Produced?
(A) Rate = K[N 2O][O], (B) Rate = K[O 2]2, (C) Rate = K[Clco][Cl 2] 2.
Related Post: