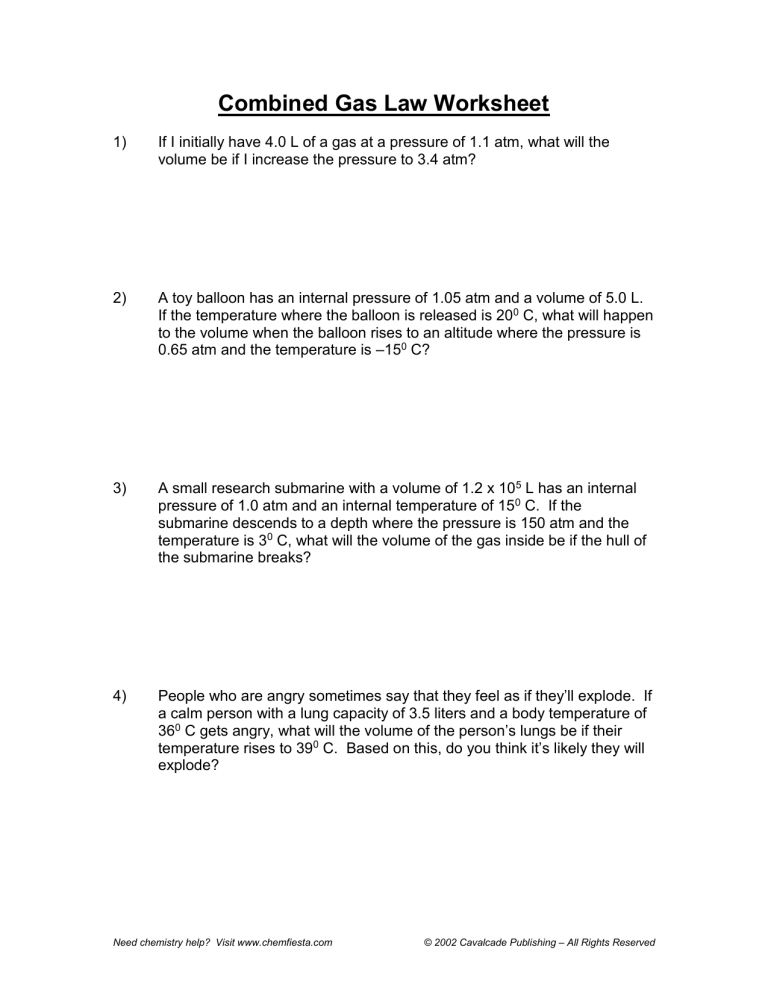
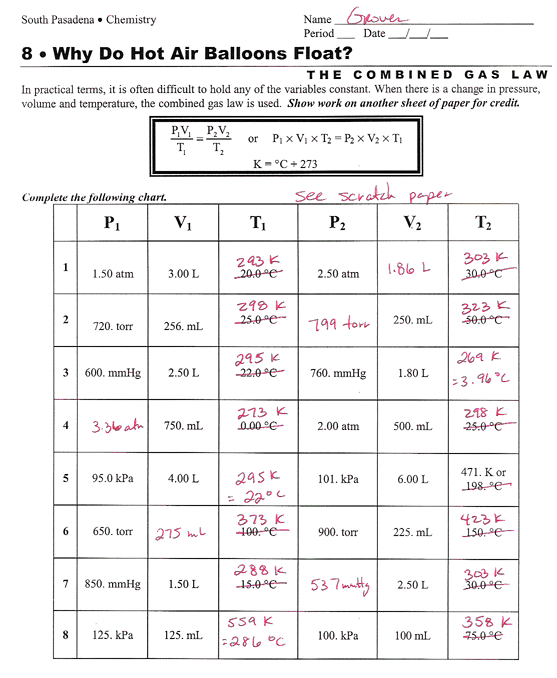
Combined Gas Law Worksheet
Combined Gas Law Worksheet - Some of the worksheets for this concept are combined gas law problems, combined gas law work, 9 23. Web up to 24% cash back the combined gas law! The correct answer is given in. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and. What will its volume be. Use the combined gas law to solve the following problems: For a combined gas law. A 952 cm3 container of gas is exerting a pressure of 108 kpa while at a temperature of 48 °c. Web combined gas law problems: If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of. Web curated and reviewed by lesson planet. Web solutions gases 1 (worksheet) “.it was as though scales fell from my eyes, doubt vanished, and was replaced by a feeling of peaceful calm.” lothar meyer, upon hearing. Web combined gas law problems: You can do the exercises online or download the worksheet as pdf. What will its volume be. 1) if i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and. Gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is 740.0 mm of mercury. Combine your two proportionality expressions for boyle’s law and for charles’ law into. Web curated and reviewed by lesson. Web up to 24% cash back the combined gas law! The rearrangement looks like this: Web combined gas law worksheet 1.1 atm, 2) a toy balloon has an internal pressure of 1.05 atm and a volume of 5.0 l. Web learn and apply the combined gas law. Some of the worksheets for this concept are combined gas law problems, combined. Web learn and apply the combined gas law. Web the form of the combined gas law most often used is this: Web solutions gases 1 (worksheet) “.it was as though scales fell from my eyes, doubt vanished, and was replaced by a feeling of peaceful calm.” lothar meyer, upon hearing. Boyle’s and charles’s law boyle’s law : You can do. Web learn and apply the combined gas law. Web the combined gas law investigates the relationship between pressure, temperature, and volume of gases; Web combined gas law worksheet 1.1 atm, 2) a toy balloon has an internal pressure of 1.05 atm and a volume of 5.0 l. Relates pressure and volume at constant temperature. If the temperature where the balloon. A 952 cm3 container of gas is exerting a pressure of 108 kpa while at a temperature of 48 °c. Web the combined gas law investigates the relationship between pressure, temperature, and volume of gases; Web the combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. You can do the. (p1v1) / t1= (p2v2) / t2. Web curated and reviewed by lesson planet. Combined gas law and ideal gas law name______________ 1. You can do the exercises online or download the worksheet as pdf. Use the combined gas law to solve the following problems: Combined gas law and ideal gas law name______________ 1. 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc. If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of. You can do the exercises online or download the worksheet as pdf. Web the form of the. Relates pressure and volume at constant temperature. Gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is 740.0 mm of mercury. Web the combined gas law! If the temperature where the balloon is released is 20° c,. Web the combined gas law expresses the relationship between the pressure, volume, and absolute temperature. Most commonly v2is being solved for. Some of the worksheets for this concept are combined gas law problems, combined gas law work, 9 23. Web learn and apply the combined gas law. The correct answer is given in. In this combined gas law worksheet, high schoolers use the temperature, the pressure and the volume of gases to find the. Web the form of the combined gas law most often used is this: (p1v1) / t1= (p2v2) / t2. Web combined gas law worksheet 1.1 atm, 2) a toy balloon has an internal pressure of 1.05 atm and a volume of 5.0 l. Combine your two proportionality expressions for boyle’s law and for charles’ law into. The rearrangement looks like this: Web carry out the following steps to derive equations for the combined gas law. Use the combined gas law to solve the following problems: A 952 cm3 container of gas is exerting a pressure of 108 kpa while at a temperature of 48 °c. Web the combined gas law investigates the relationship between pressure, temperature, and volume of gases; One thing we notice about all the gas laws is that, collectively, volume and pressure are always in the numerator, and temperature is. Web combined gas law problems: If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of. For a combined gas law. A gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is. 1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc. Web up to 24% cash back the combined gas law! Web solutions gases 1 (worksheet) “.it was as though scales fell from my eyes, doubt vanished, and was replaced by a feeling of peaceful calm.” lothar meyer, upon hearing. Use the combined gas law to solve the following problems: Web learn and apply the combined gas law. Combined gas law and ideal gas law name______________ 1. A 952 cm3 container of gas is exerting a pressure of 108 kpa while at a temperature of 48 °c. You can do the exercises online or download the worksheet as pdf. Web the ideal and combined gas laws pv = nrt or p1v1 = p2v2 t1 t2 use your knowledge of the ideal and combined gas laws to solve the following problems. Use the combined gas law to solve the following problems: Combine your two proportionality expressions for boyle’s law and for charles’ law into. One thing we notice about all the gas laws is that, collectively, volume and pressure are always in the numerator, and temperature is. Web curated and reviewed by lesson planet. Web combined gas law problems: Gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is 740.0 mm of mercury. What will its volume be. Use the combined gas law to solve the following problems: The rearrangement looks like this: Web the combined gas law investigates the relationship between pressure, temperature, and volume of gases; Relates pressure and volume at constant temperature. Some of the worksheets for this concept are combined gas law problems, combined gas law work, 9 23. If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of.Gas Laws Worksheet 2 Boyles Charles and Combined Gases Pressure
Combined Gas Law Worksheet 1
Quiz & Worksheet Combined Gas Law
Gas Law Practice Worksheet Free Download Goodimg.co
Foothill High School
Worksheet Gas Laws Chemistry At Central High School Worksheet
Gen Chem Page
8 Combined Gas Law Worksheet /
Combined Gas Law Problems Worksheet —
Combined Gas Law Worksheet
Web Carry Out The Following Steps To Derive Equations For The Combined Gas Law.
A Gas Balloon Has A Volume Of 106.0 Liters When The Temperature Is 45.0 °C And The Pressure Is.
Web The Combined Gas Law!
In This Combined Gas Law Worksheet, High Schoolers Use The Temperature, The Pressure And The Volume Of Gases To Find The.
Related Post: