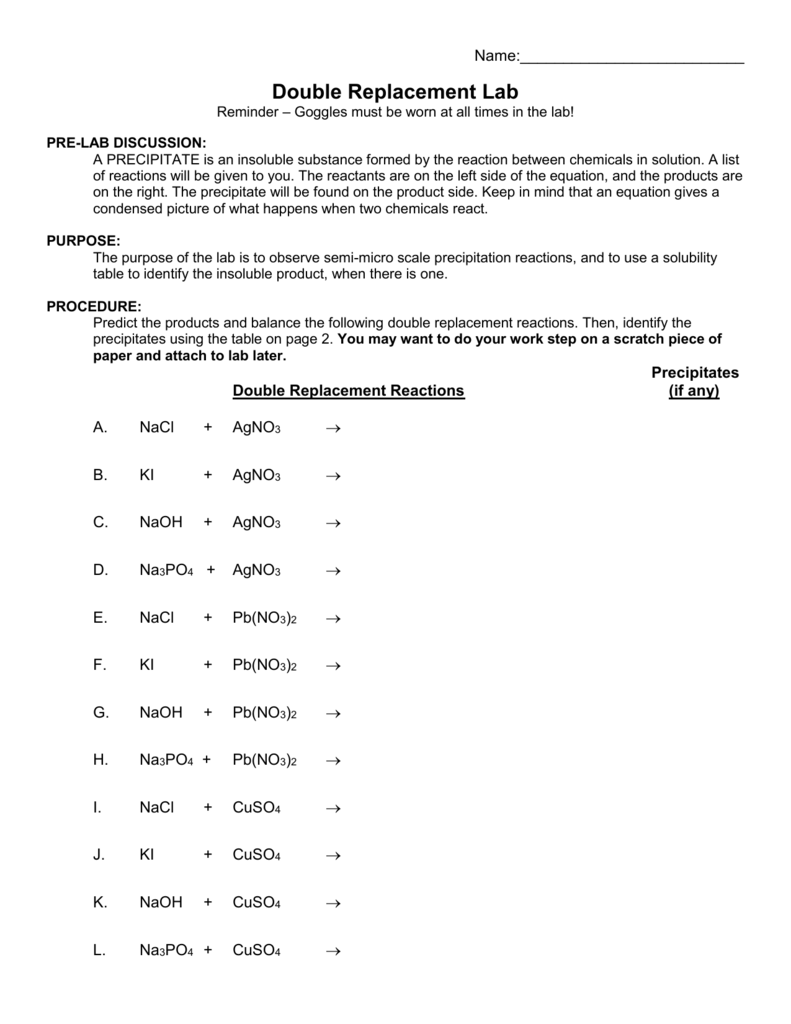
Double Replacement Reaction Worksheet Answers
Double Replacement Reaction Worksheet Answers - Diatomic elements do not count; Web the cations and anions of two compounds switch places to form two new products in a double replacement or double displacement reaction. Type of reaction reactants products will a precipitate form for the following. Types are chemical reaction worksheet ch. The students may be asked to identify their unknown solution containing a metal by testing. An unknown solution containing the ions the students have used may be prepared. Web to rest exist up to you!! You do not need to worry about balancing the equation. It is important that the formulas of the products be written correctly. Write correct formulas for the products in these double replacement reactions. Included in the chemistry instructor resources subscription. Web the cations and anions of two compounds switch places to form two new products in a double replacement or double displacement reaction. An unknown solution containing the ions the students have used may be prepared. Type of reaction reactants products will a precipitate form for the following. Double replacement reactions fall 2017. A double replacement reaction is a type of chemical reaction where two reactants exchange. Write correct formulas for the items in these double. List predicting products for single & double replacement reactions created by teaching elements use this resource for a fun way to help students practice predicting products for single & double replacement reactions. Identify the products for each. 3 nabr + 1 h3po4 na3po4 + 3 hbr ca(oh)2 + 1 al2(so4)3 type of reaction: Web to rest exist up to you!! The hydrogen atoms in hcl are replaced by zn atoms, and in the process a new element—hydrogen—is formed. Double replacement reactions fall 2017 page 3 of 9 when we pour the two solutions together we have four. Just be sure that the new pairs come out with the positive ion named first, and paired with a negative ion. E) li₂n +_3nh₂nolino3+ (nh4) n reaction type: Web the cations and anions of two compounds switch places to form two new products in a double replacement or double displacement reaction. Web single and double replacement reactions worksheet directions: Write. Web the cations and anions of two compounds switch places to form two new products in a double replacement or double displacement reaction. The students may be asked to identify their unknown solution containing a metal by testing. Types are chemical reaction worksheet ch. E) li₂n +_3nh₂nolino3+ (nh4) n reaction type: Web view double replacement reactions worksheet answers.pdf from chemistry. All double replacement reactions have the general form: The hydrogen atoms in hcl are replaced by zn atoms, and in the process a new element—hydrogen—is formed. It is important that the formulas of the products be written correctly. Web single and double replacement reactions worksheet directions: Identify the products for each of the following reactions below. They are included in the single replacement category. Web 380 + results sort by: E) li₂n +_3nh₂nolino3+ (nh4) n reaction type: Web the cations and anions of two compounds switch places to form two new products in a double replacement or double displacement reaction. Reactions that can be classified as double replacements include precipitation reactions, neutralization reactions and gas forming. Type of reaction reactants products will a precipitate form for the following. Write correct formulas for the items in these double. They are included in the single replacement category. Just be sure that the new pairs come out with the positive ion named first, and paired with a negative ion. Web double replacement reactions. Just be sure that the new pairs come out with the positive ion named first, and paired with a negative ion. Web in double replacement, both reactants are compounds, each with a cation part and an anion part. A double replacement reaction is a type of chemical reaction where two reactants exchange. Web 380 + results sort by: Reactions that. Write correct formulas for the items in these double. Type of reaction reactants products will a precipitate form for the following. The students may be asked to identify their unknown solution containing a metal by testing. 3 nabr + 1 h3po4 na3po4 + 3 hbr ca(oh)2 + 1 al2(so4)3 type of reaction: Web to rest exist up to you!! Web in double replacement, both reactants are compounds, each with a cation part and an anion part. Web view double replacement reactions worksheet answers.pdf from chemistry 1 at maseno university. Write correct formulas for the items in these double. 3 nabr + 1 h3po4 na3po4 + 3 hbr ca(oh)2 + 1 al2(so4)3 type of reaction: Reactions that can be classified as double replacements include precipitation reactions, neutralization reactions and gas forming. (10.1) a b + c d → a d + c b. (remember (aq) means dissolved in water) consider the possible combinations. It is important that the formulas of the products be written correctly. A double replacement reaction is a type of chemical reaction where two reactants exchange. Type of reaction reactants products will a precipitate form for the following. Just be sure that the new pairs come out with the positive ion named first, and paired with a negative ion. The hydrogen atoms in hcl are replaced by zn atoms, and in the process a new element—hydrogen—is formed. An unknown solution containing the ions the students have used may be prepared. E) li₂n +_3nh₂nolino3+ (nh4) n reaction type: In a double replacement reactions , typically one of the products is a precipitate, a gas, or a. Identify the products for each of the following reactions below. Diatomic elements do not count; Web double replacement reactions. Write correct formulas for the products in these double replacement reactions. Web 380 + results sort by: Double displacement 3 caso4 + 2 al(oh)3 3) 4) 5) 6) 7) 8). All double replacement reactions have the general form: Web the cations and anions of two compounds switch places to form two new products in a double replacement or double displacement reaction. Web view double replacement reactions worksheet answers.pdf from chemistry 1 at maseno university. Just be sure that the new pairs come out with the positive ion named first, and paired with a negative ion. An unknown solution containing the ions the students have used may be prepared. A double replacement reaction is a type of chemical reaction where two reactants exchange. Web to rest exist up to you!! Write correct formulas for the items in these double. The worksheet may be graded along with the students’ observations. Web single and double replacement reactions worksheet directions: Web 380 + results sort by: They are included in the single replacement category. Write correct formulas for the products in these double replacement reactions. You do not need to worry about balancing the equation. (10.1) a b + c d → a d + c b.Double Replacement Reaction Worksheet
Single And Double Replacement Reactions Worksheet With Answers
️Double Replacement Reaction Worksheet Free Download Goodimg.co
chemistry double replacement reaction worksheet practice reactions
30 Chemical Bonds Worksheet Answers Education Template
30 Double Replacement Reaction Worksheet Education Template
️Worksheet 5 Double Replacement Reactions Free Download Gambr.co
Double Replacement Reactions Worksheet Answer Key 28 Double
30 Double Replacement Reaction Worksheet Education Template
Single And Double Replacement Reactions Worksheet Answers Rocco Worksheet
E) Li₂N +_3Nh₂Nolino3+ (Nh4) N Reaction Type:
3 Nabr + 1 H3Po4 Na3Po4 + 3 Hbr Ca(Oh)2 + 1 Al2(So4)3 Type Of Reaction:
Included In The Chemistry Instructor Resources Subscription.
Web In Double Replacement, Both Reactants Are Compounds, Each With A Cation Part And An Anion Part.
Related Post: