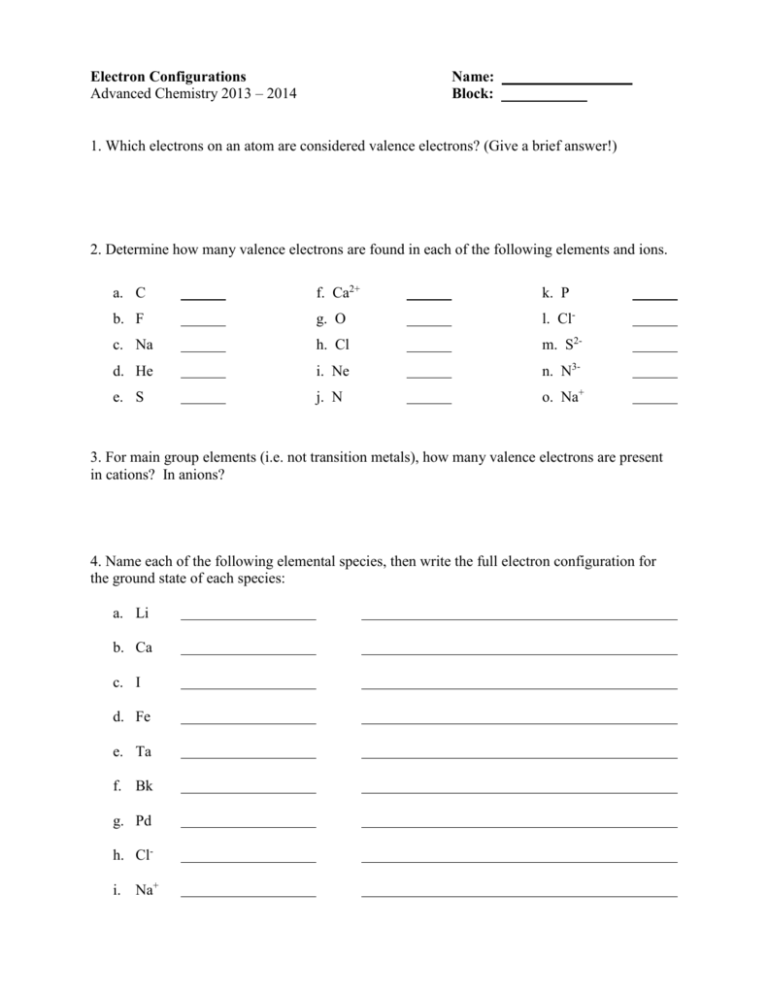
Electron Configuration Chem Worksheet 5-6
Electron Configuration Chem Worksheet 5-6 - Ap chemistry chapter 5 worksheet # 3 name _____ 1. Web this worksheet from teach simple tests students’ knowledge of standard and noble gas electron configuration, how to read configurations, and find mistakes in them. A typical electron configuration consists of numbers, letters, and superscripts with the following format: The orbital designation is followed by a superscript number that tells how many electrons are found in that orbital. Draw the electron configuration for the outer shell only for those elements in #5: Suitable for grade 8 upward. Web orbital with 5 electrons. 1 s^2 2 s^2 1s22s2 a 1 s^2 2 s^2 1s22s2 1 s^2 2 s^1 2p^1 1s22s12p1 b 1 s^2 2 s^1 2p^1 1s22s12p1 1 s^2 2 p^2 1s22p2 c 1 s^2 2 p^2 1s22p2 1 s^2 1 p^2 1s21p2 d Types of bonds (ionic bonding) by palmergm: The electron configurations and orbital diagrams of these four elements are: Get everything done in minutes. A letter indicates the type of orbital; These numbers represent electrons and are noted as superscripts in the electron configuration. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Web to. The alkali metal sodium (atomic number 11) has one more electron than the neon atom. The electron configurations and orbital diagrams of these four elements are: Web orbital with 5 electrons. Write the ground state electron configuration for neutral titanium. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals. Web an electron configuration is a method of indicating the arrangement of electrons about a nucleus. Web to know the electron configuration, note all of the subshells that you passed on your way to boron, which in this case, would be 1s, 2s, and 2p. Web help your students learn how to write electron orbital diagrams and electron configurations with. The following designation represents an atom with electrons ex. Web to know the electron configuration, note all of the subshells that you passed on your way to boron, which in this case, would be 1s, 2s, and 2p. These numbers represent electrons and are noted as superscripts in the electron configuration. Web all of the electrons in the noble gas. The orbital designation is followed by a superscript number that tells how many electrons are found in that orbital. Write the ground state electron configuration for neutral titanium. Web orbital with 5 electrons. Web an electron configuration is a method of indicating the arrangement of electrons about a nucleus. G9isrscience member for 1 year 11 months. A typical electron configuration consists of numbers, letters, and superscripts with the following format: Electron configurations can be abbreviated by writing the element symbol for the previous noblegas in brackets, followed by the remainingelectrons. 1 s^2 2 s^2 1s22s2 a 1 s^2 2 s^2 1s22s2 1 s^2 2 s^1 2p^1 1s22s12p1 b 1 s^2 2 s^1 2p^1 1s22s12p1 1 s^2. Some of the worksheets for this concept are electron configuration practice work, electron configuration work, electron configuration practice work, chemistry 143 electron configurations caddell, atomic structure and electron configurations multiple, exam 4 collected essays, 13. Web electron configuration electron configuration id: The easiest and most reliable technique for writing electron configurations is to use the periodic table as your guide.. Web complete electron configuration chem worksheet 5 6 answer key online with us legal forms. A typical electron configuration consists of numbers, letters, and superscripts with the following format: Web an electron configuration is a method of indicating the arrangement of electrons about a nucleus. The orbital designation is followed by a superscript number that tells how many electrons are. Web an electron configuration is a method of indicating the arrangement of electrons about a nucleus. Web electron configuration electron configuration id: Give the electron configuration of sulfur, giving the number of electrons in each subshell. Types of bonds (ionic bonding) by palmergm: Electron structure share / print worksheet. Give the electron configuration of sulfur, giving the number of electrons in each subshell. Web complete electron configuration chem worksheet 5 6 answer key online with us legal forms. Types of bonds (ionic bonding) by palmergm: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. G9isrscience member for 1 year 11 months. Draw the electron configuration for the outer shell only for those elements in #5: Check out how easy it is to complete and esign documents online using fillable templates and a powerful editor. Web this worksheet from teach simple tests students’ knowledge of standard and noble gas electron configuration, how to read configurations, and find mistakes in them. For each of the following, show the outer shell orbital notation, outer shell electron configuration, and the electron dot diagram: The following designation represents an atom with electrons ex. Ap chemistry chapter 5 worksheet # 3 name _____ 1. Now, how many elements did you pass in each block? An answer scheme is provided to allow for peer marking. Introduction to the periodic table by drjritchie: Save or instantly send your ready documents. These numbers represent electrons and are noted as superscripts in the electron configuration. The electron configurations in this worksheet assume that lanthanum (la) is the first element in the 4f block and that actinium (ac) is the first element in the 5f block. Web determine if the following electron configurations are correct: Write the ground state electron configuration for neutral titanium. The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. A number indicates the energy level (the number is called the principal quantum number.). Web to know the electron configuration, note all of the subshells that you passed on your way to boron, which in this case, would be 1s, 2s, and 2p. 19) 1s22s22p63s23p64s24d104p5 not valid (take a look at “4d”) 20) 1s22s22p63s23d5 not valid (3p comes after 3s) 21) [ra] 7s25f8 not valid (radium isn’t a noble gas) 22) [xe] not valid (an element can’t be its own electron configuration) Web give the electron configuration of sodium, giving the number of electrons in each subshell. Get everything done in minutes. Types of bonds (ionic bonding) by palmergm: Web help your students learn how to write electron orbital diagrams and electron configurations with this long, detailed worksheet set!this worksheet set includes 6+ pages of problems for students to complete, including completed examples and a complete answer key (pictured above). Check out how easy it is to complete and esign documents online using fillable templates and a powerful editor. For example, rather than writing all of the electrons in antimony (element 51), the first 36electrons are represented by [kr]. The electron configurations in this worksheet assume that lanthanum (la) is the first element in the 4f block and that actinium (ac) is the first element in the 5f block. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Write the ground state electron configuration for neutral hydrogen and then write the electron configuration for an excited state of hydrogen. Web complete electron configuration chem worksheet 5 6 answer key online with us legal forms. The electron configurations and orbital diagrams of these four elements are: The electron configurations and orbital diagrams of these four elements are: 1 s^2 2 s^2 1s22s2 a 1 s^2 2 s^2 1s22s2 1 s^2 2 s^1 2p^1 1s22s12p1 b 1 s^2 2 s^1 2p^1 1s22s12p1 1 s^2 2 p^2 1s22p2 c 1 s^2 2 p^2 1s22p2 1 s^2 1 p^2 1s21p2 d A number indicates the energy level (the number is called the principal quantum number.). Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Some of the worksheets for this concept are electron configuration practice work, electron configuration work, electron configuration practice work, chemistry 143 electron configurations caddell, atomic structure and electron configurations multiple, exam 4 collected essays, 13. Web this is a great teaching worksheet that can be used to teach about electron configurations, energy levels, energy sub levels, by filling in the circles with arrows or an x student are able to come up with the electron configuration of atoms by simple knowing how many electrons an atom has and following the aufbau principle of lowest energy.Electron Configuration Chem Worksheet 5 6 Answers —
Electron Configuration Chem Worksheet 5 6 Answers
Electron Configurations Pacticew Worksheet With Key Electron
Electron Configuration Chem Worksheet 5 6 Answers —
Electron Configuration Chem Worksheet 56
Electron Configuration Worksheet Answer Key / 5 6electronconfig
Electron Configuration worksheet
Electron Configurations Worksheet Answer Key
Electron Configuration Practice Worksheet
Electron Configuration Chem Worksheet 5 6 Answers —
The Orbital Designation Is Followed By A Superscript Number That Tells How Many Electrons Are Found In That Orbital.
The Following Designation Represents An Atom With Electrons Ex.
These Numbers Represent Electrons And Are Noted As Superscripts In The Electron Configuration.
Web These Electron Configurations Have Mistakes, Determine What Is Wrong.
Related Post: