Empirical Formula Worksheet 1
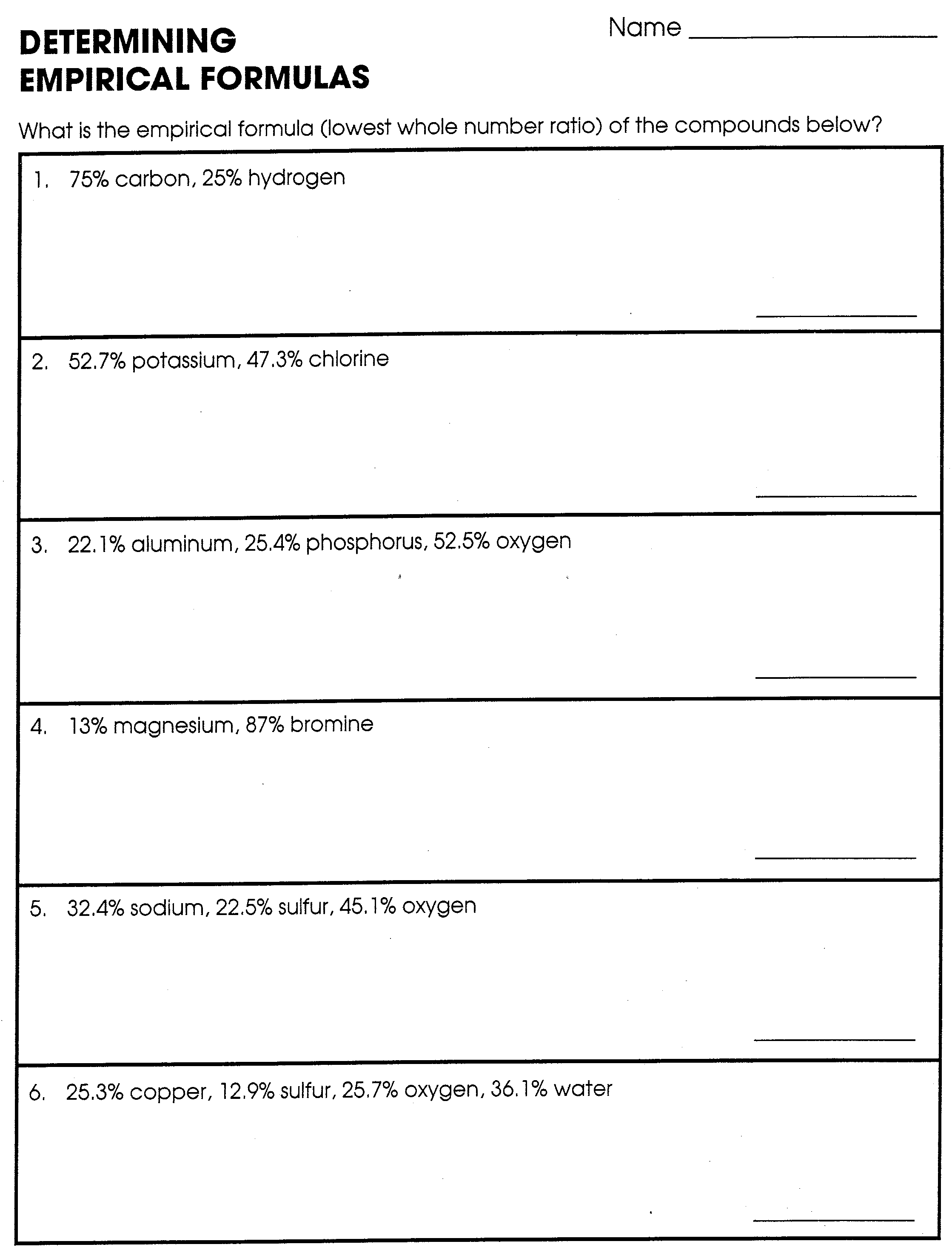
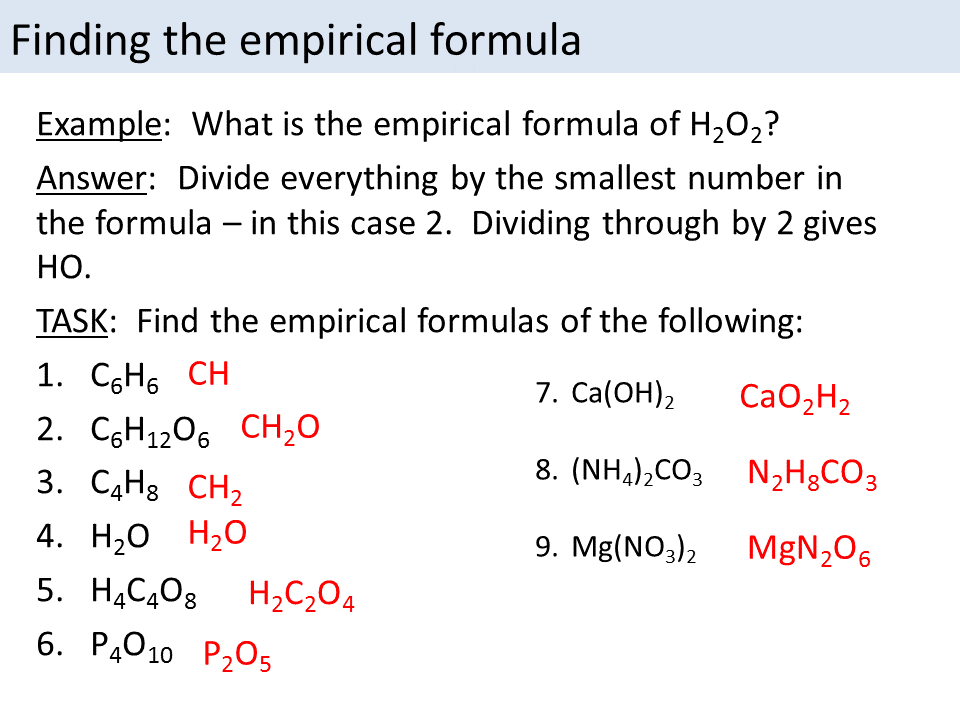
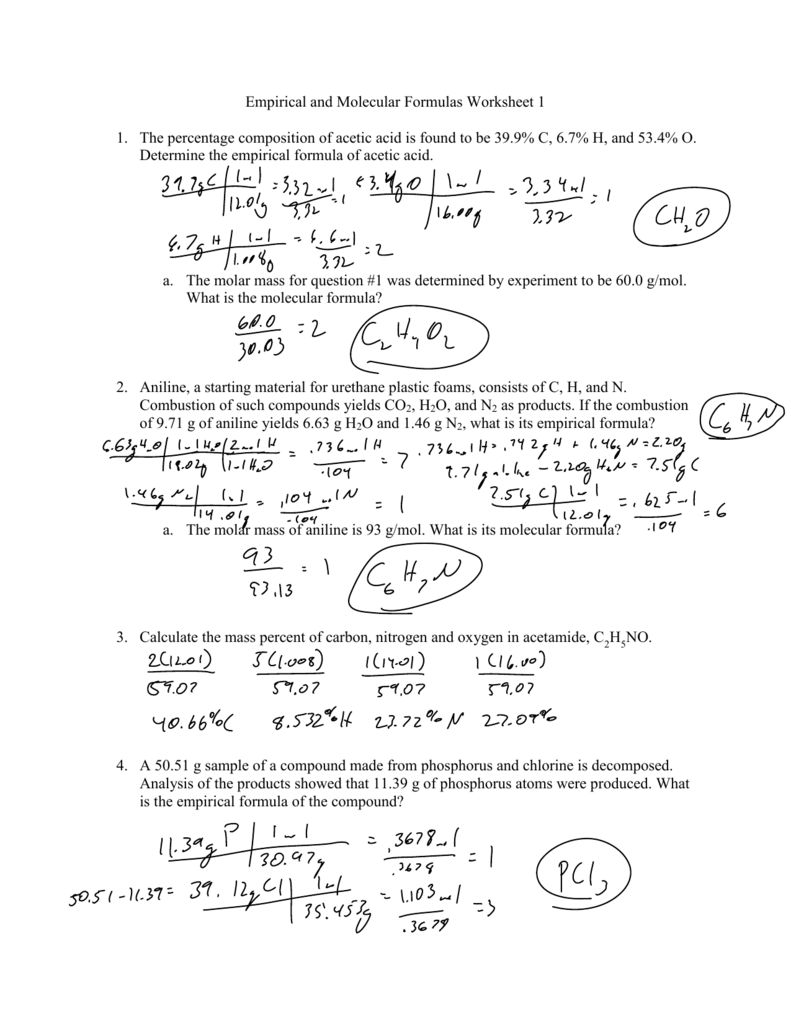
Empirical Formula Worksheet 1 - Web an empirical formula tells us the relative ratios of different atoms in a compound. Web recall that empirical formulas are symbols representing the relative numbers of a compound’s elements. Web empirical formula worksheet 1. Find the empirical formula and name for each of the following. D) a compound analyzes as 79.08 % c; Thus, h 2 o is composed of. The molecular formula is an actual, real formula. Empirical_formula_worksheet.pdf — pdf document, 1.83 mb (1921394 bytes) 5.54 % h and 15.38 % n. Web this activity worksheet examines both empirical formula and molecular formula through calculations and scaffolding steps. Web an empirical formula is the lowest common ratio between the elements in a compound. This quiz/worksheet combo will help test your understanding of. Convert the grams of each element to moles. Write the empirical formula for the following compounds. Web empirical and molecular formula worksheet show work on a separate sheet of paper. In order to calculate a molecular. The molecular formula is an actual, real formula. Find the empirical formula of a compound. Thus, h 2 o is composed of. Determining the absolute numbers of atoms that compose a. The ratios hold true on the molar level as well. Web empirical formula worksheet 1. Web empirical and molecular formula worksheet show work on a separate sheet of paper. A compound is 24.7% calcium, 1.2%. Web an empirical formula tells us the relative ratios of different atoms in a compound. Web up to $3 cash back empirical formulas worksheet, #1 directions: Web recall that empirical formulas are symbols representing the relative numbers of a compound’s elements. Web this activity worksheet examines both empirical formula and molecular formula through calculations and scaffolding steps. Determining the absolute numbers of atoms that compose a. Web the empirical formula of a compound is the. Write the empirical formula for the following compounds. Find the empirical formula and name for each of the following. 5.54 % h and 15.38 % n. A compound is 24.7% calcium, 1.2%. Web emperical formula worksheet 1. Determining the absolute numbers of atoms that compose a. What is the empirical formula for a compound which contains 0.0134 g of iron, 0.00769 g of sulfur and 0.0115 g of oxygen? Web what is the empirical formula? In order to calculate a molecular. A compound is 24.7% calcium, 1.2%. A compound is 24.7% calcium, 1.2%. What is the empirical formula for a compound which contains 0.0134 g of iron, 0.00769 g of sulfur and 0.0115 g of oxygen? 5.54 % h and 15.38 % n. Web the empirical formula of a compound is the simplest whole number ratio of each type of atom in a compound. Find the empirical. Find the empirical formula for a compound which contains 32.8% chromium and 67.2% chlorine. Web the empirical formula of a compound is the simplest whole number ratio of each type of atom in a compound. Web recall that empirical formulas are symbols representing the relative numbers of a compound’s elements. Convert the grams of each element to moles. 5.54 %. Find the empirical formula of a compound that contains 56.3% p and 43.7 % o. 1) c 6h 6 2). 6 % mass mass mole s divide by small multiply until whole final empirical ratio element mass molar mass moles mole ratio show your work for the. Thus, h 2 o is composed of. Convert the grams of each element. Web recall that empirical formulas are symbols representing the relative numbers of a compound’s elements. The ratios hold true on the molar level as well. What is the empirical formula for a compound which contains 0.0134 g of iron, 0.00769 g of sulfur and 0.0115 g of oxygen? Web an empirical formula is the lowest common ratio between the elements. Find the empirical formula and name for each of the following. Empirical_formula_worksheet.pdf — pdf document, 1.83 mb (1921394 bytes) Web to print or download this file, click the link below: 6 % mass mass mole s divide by small multiply until whole final empirical ratio element mass molar mass moles mole ratio show your work for the. Web an empirical formula is the lowest common ratio between the elements in a compound. What is the empirical formula for a compound which contains 0.0134 g of iron, 0.00769 g of sulfur and 0.0115 g of oxygen? Web the empirical formula of a compound is the simplest whole number ratio of each type of atom in a compound. Write the empirical formula for the following compounds. 1) c 6h 6 2). Web determine the empirical formula from the percent composition for each of the following: Web recall that empirical formulas are symbols representing the relative numbers of a compound’s elements. In order to calculate a molecular. 5.54 % h and 15.38 % n. Find the empirical formula of a compound that contains 56.3% p and 43.7 % o. Web up to $3 cash back empirical formulas worksheet, #1 directions: Web empirical formula worksheet 1. Determining the absolute numbers of atoms that compose a. Web this activity worksheet examines both empirical formula and molecular formula through calculations and scaffolding steps. Web empirical formula worksheet. The molecular formula is an actual, real formula. A compound is 24.7% calcium, 1.2%. 5.54 % h and 15.38 % n. Find the empirical formula and name for each of the following. D) a compound analyzes as 79.08 % c; 6 % mass mass mole s divide by small multiply until whole final empirical ratio element mass molar mass moles mole ratio show your work for the. Web empirical formula worksheet 1. Write the empirical formula for the following compounds. Web to print or download this file, click the link below: Web up to $3 cash back empirical formulas worksheet, #1 directions: Empirical_formula_worksheet.pdf — pdf document, 1.83 mb (1921394 bytes) What is the empirical formula for a compound which contains 0.0134 g of iron, 0.00769 g of sulfur and 0.0115 g of oxygen? Find the empirical formula of a compound that contains 56.3% p and 43.7 % o. Find the empirical formula for a compound which contains 32.8% chromium and 67.2% chlorine. Web an empirical formula tells us the relative ratios of different atoms in a compound. Web the empirical formula of a compound is the simplest whole number ratio of each type of atom in a compound. Find the empirical formula of a compound.Empirical and Molecular Formulas Worksheet 1 1. The percentage
Empirical And Molecular Formula Worksheet Solved Percent Composition
empirical formula worksheet 1
Pin on Chemistry Lecture Notes
List Of Empirical Formulas Worksheet References Alec Worksheet
Chemistry Empirical Formula Worksheets
Empirical Formula Worksheet Answers With Work Organicfer
empirical formula worksheet
Empirical And Molecular Formula Worksheet printable pdf download
Empirical Formula Worksheet 1 Answers Pdf Master of
Students First Calculate Empirical Formula Using Percentages And Then Progress Into Calculating Molecular Formula Through Examples.
Determining The Absolute Numbers Of Atoms That Compose A.
Thus, H 2 O Is Composed Of.
Web An Empirical Formula Is The Lowest Common Ratio Between The Elements In A Compound.
Related Post:


.PNG)