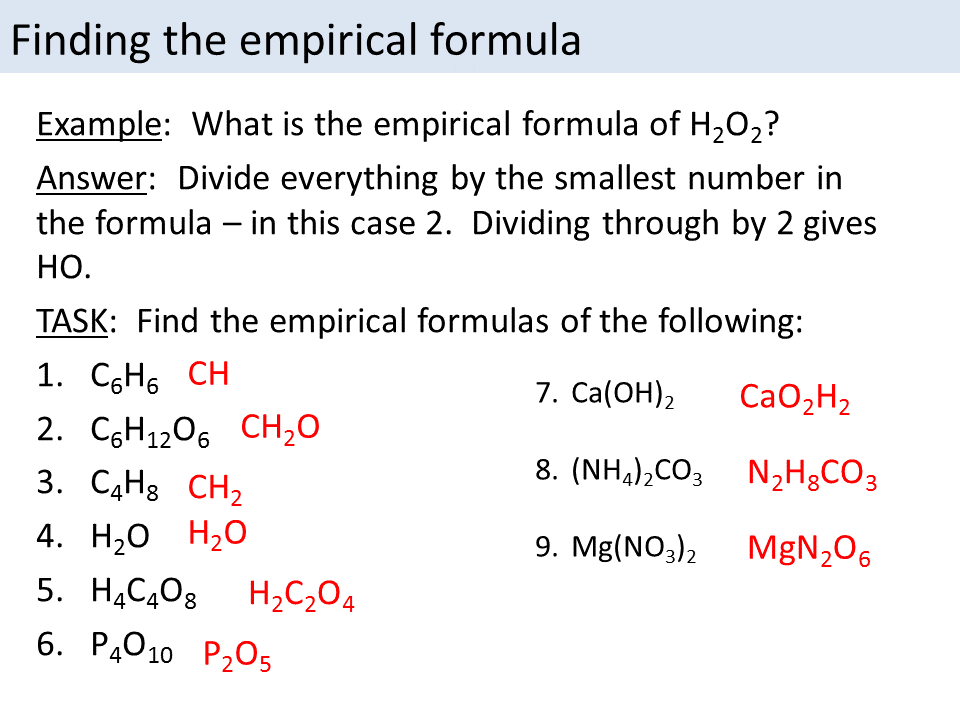
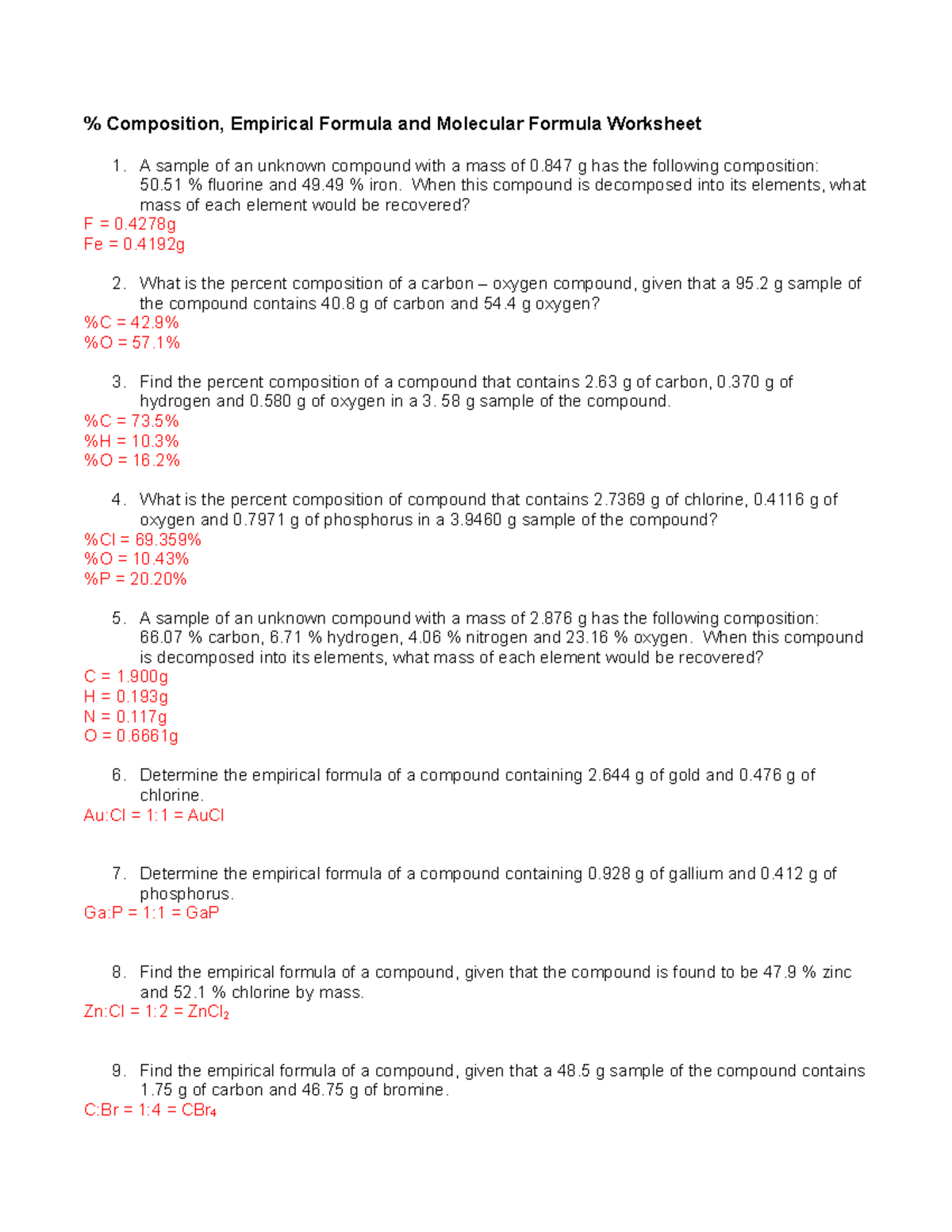
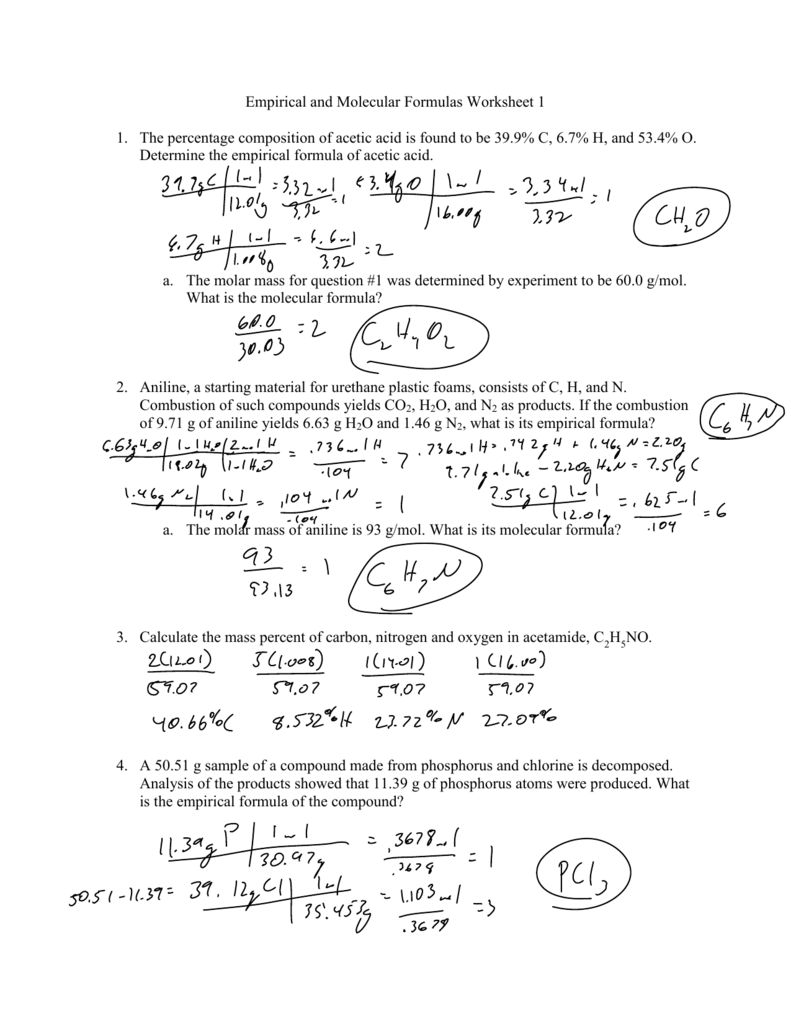
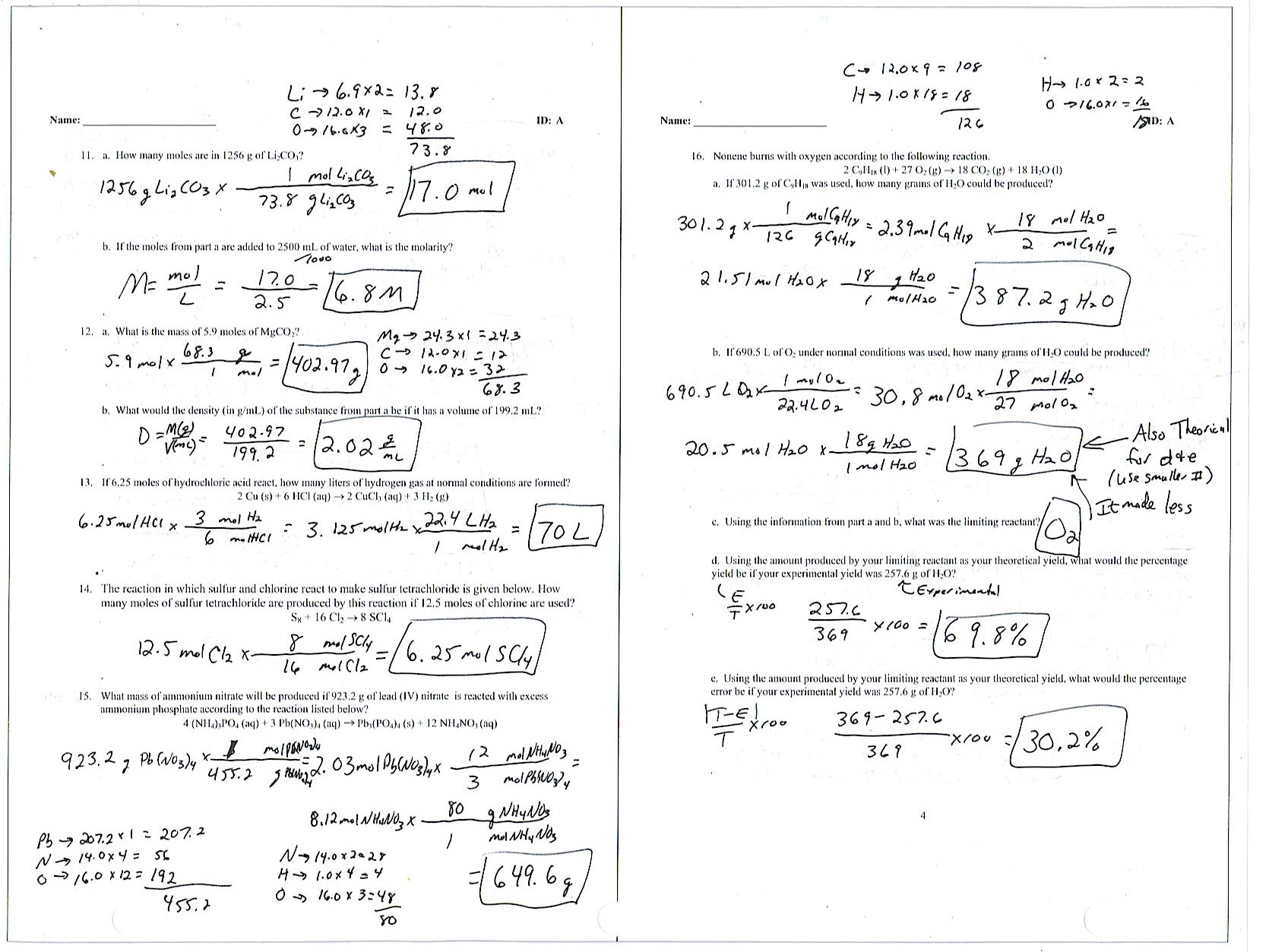
Empirical/Molecular Formula Practice Worksheet Answers
Empirical/Molecular Formula Practice Worksheet Answers - C 3 h 4 o 2; Empirical/molecularformulapractice worksheet directions:find the empirical and molecular formulas given the percent. To determine the molecular formula of a compound, you must know the compound’s molar mass. Divide the molecular mass by the empirical formula mass to determine. 1) c6h6 c 3 h 3 6) c8h18 c 4 h 9 7) wo2 wo 2 8). Calculating empirical & molecular formulas 1. Find the empirical and molecular formulas given the percent compositions or masses. Web determine the empirical formula for: What’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? Web empirical/molecular formula practice worksheet directions: Web determine the empirical formula for: Determine the empirical formula for: The molecular formula is an actual, real formula. Find the empirical and molecular formulas given the. Calculating empirical & molecular formulas 1. Find the empirical and molecular formulas given the percent compositions or masses. Web 1) what is the empirical formula of a compound that contains 0.783g of carbon, 0.196g of hydrogen and 0.521g of oxygen? Web an empirical formula is the lowest common ratio between the elements in a compound. Ca 3 p 2 o 8 ;. The molecular formula is. Find the empirical and molecular formulas given the percent compositions or masses. 42.07g na, 18.89g p, and 39.04g o, determine the empirical formula. Web 1) what is the empirical formula of a compound that contains 0.783g of carbon, 0.196g of hydrogen and 0.521g of oxygen? Empirical/molecularformulapractice worksheet directions:find the empirical and molecular formulas given the percent. Web an empirical formula. What’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? Web an empirical formula is the lowest common ratio between the elements in a compound. In order to calculate a molecular. Empirical/molecularformulapractice worksheet directions:find the empirical and molecular formulas given the percent. Divide the molecular mass by the empirical formula mass to determine. Web empirical/molecular formula practice worksheet directions: The molecular formula is an actual, real formula. In order to calculate a molecular. What is the empirical formula if you have 36.84% nitrogen and 63.16% oxygen? A periodic table will be required to complete this practice test. Web empirical/molecular formula practice worksheet directions: A periodic table will be required to complete this practice test. Find the empirical and molecular formulas given the percent compositions or masses. Determine the empirical formula for: To determine the molecular formula of a compound, you must know the compound’s molar mass. Ca 3 p 2 o 8 ;. Web determine the empirical formula for: Web an empirical formula is the lowest common ratio between the elements in a compound. The empirical formula for the compound having the formula h2c2o4 is [a] c2h2 [b] co2h [c] coh [d] c2o4h2 [e]. Empirical/molecular formula practice worksheet directions: Web empirical and molecular formulas worksheet teaching resources | tpt results for empirical and molecular formulas worksheet 154 results sort by: 1) c6h6 c 3 h 3 6) c8h18 c 4 h 9 7) wo2 wo 2 8). 2) what is empirical formula of a compound which. Web empirical/molecular formula practice worksheet directions: 42.07g na, 18.89g p, and 39.04g o,. A periodic table will be required to complete this practice test. Web determine the empirical formula of a compound that contains 50.85 % carbon, 8.47 % hydrogen and 40.68 % oxygen c h mass (g) 50.85 8.47 40.68 molar mass (g/mol) 12.01. Ca 3 p 2 o 8 ;. Web empirical and molecular formulas worksheet teaching resources | tpt results. What is the empirical formula if you have 36.84% nitrogen and 63.16% oxygen? To determine the molecular formula of a compound, you must know the compound’s molar mass. Find the empirical and molecular formulas given the percent compositions or masses. Empirical/molecular formula practice worksheet directions: Calculating empirical & molecular formulas 1. To determine the molecular formula of a compound, you must know the compound’s molar mass. Web empirical and molecular formula worksheet answer key write the empirical formula for the following compounds. What’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? Empirical/molecularformulapractice worksheet directions:find the empirical and molecular formulas given the percent. Find the empirical and molecular formulas given the percent compositions or masses. Web determine the empirical formula for: Divide the molecular mass by the empirical formula mass to determine. Web an empirical formula is the lowest common ratio between the elements in a compound. Web empirical and molecular formulas worksheet teaching resources | tpt results for empirical and molecular formulas worksheet 154 results sort by: Find the empirical and molecular formulas given the. Find the empirical and molecular formulas given the percent compositions or masses. Empirical/molecular formula practice worksheet directions: Empirical/molecular formula practice worksheet directions: Calculating empirical & molecular formulas 1. The molecular formula is an actual, real formula. Determine the empirical formula for: 42.07g na, 18.89g p, and 39.04g o, determine the empirical formula. Web empirical/molecular formula practice worksheet directions: C 3 h 4 o 2; If the molar mass of the compound in problem 1 is 110. 42.07g na, 18.89g p, and 39.04g o, determine the empirical formula. Web determine the empirical formula of a compound that contains 50.85 % carbon, 8.47 % hydrogen and 40.68 % oxygen c h mass (g) 50.85 8.47 40.68 molar mass (g/mol) 12.01. 1) c6h6 c 3 h 3 6) c8h18 c 4 h 9 7) wo2 wo 2 8). Find the empirical and molecular formulas given the. If the molar mass of the compound in problem 1 is 110. Divide the molecular mass by the empirical formula mass to determine. Determine the empirical formula for: Find the empirical and molecular formulas given the percent compositions or masses. Empirical/molecular formula practice worksheet directions: Calculating empirical & molecular formulas 1. To determine the molecular formula of a compound, you must know the compound’s molar mass. Ca 3 p 2 o 8 ;. The molecular formula is an actual, real formula. A periodic table will be required to complete this practice test. C 3 h 4 o 2; In order to calculate a molecular.Chemistry Unit 4 Worksheet 2 Answers
Empirical Formula Worksheet 1 Answers Pdf Master of
13 Best Images of AP Chemistry Empirical Formula Worksheet Molecular
Empirical/Molecular Formula Practice Worksheets Answer Key
12 Best Images of Chemistry Mole Practice Worksheet Mole Calculation
Empirical And Molecular Formulas Worksheet
Molecular Mass And Percent Composition Worksheet Answers
Chemistry Empirical Formula Worksheets
Chemistry Empirical Formula Worksheets
Empirical Molecular Formula Worksheet Answers Econed
Web Empirical And Molecular Formulas Worksheet Teaching Resources | Tpt Results For Empirical And Molecular Formulas Worksheet 154 Results Sort By:
The Empirical Formula For The Compound Having The Formula H2C2O4 Is [A] C2H2 [B] Co2H [C] Coh [D] C2O4H2 [E].
Web An Empirical Formula Is The Lowest Common Ratio Between The Elements In A Compound.
Empirical/Molecularformulapractice Worksheet Directions:find The Empirical And Molecular Formulas Given The Percent.
Related Post: