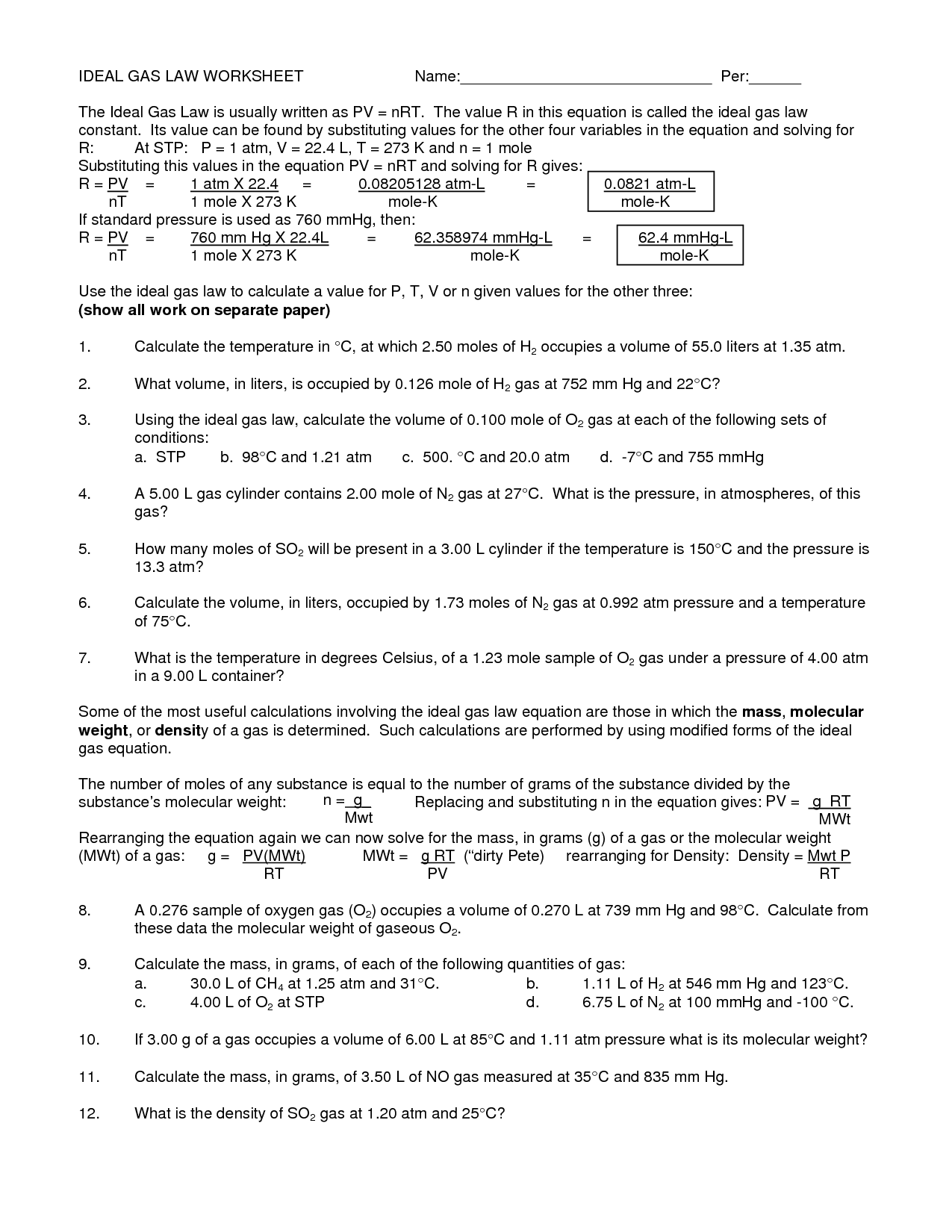
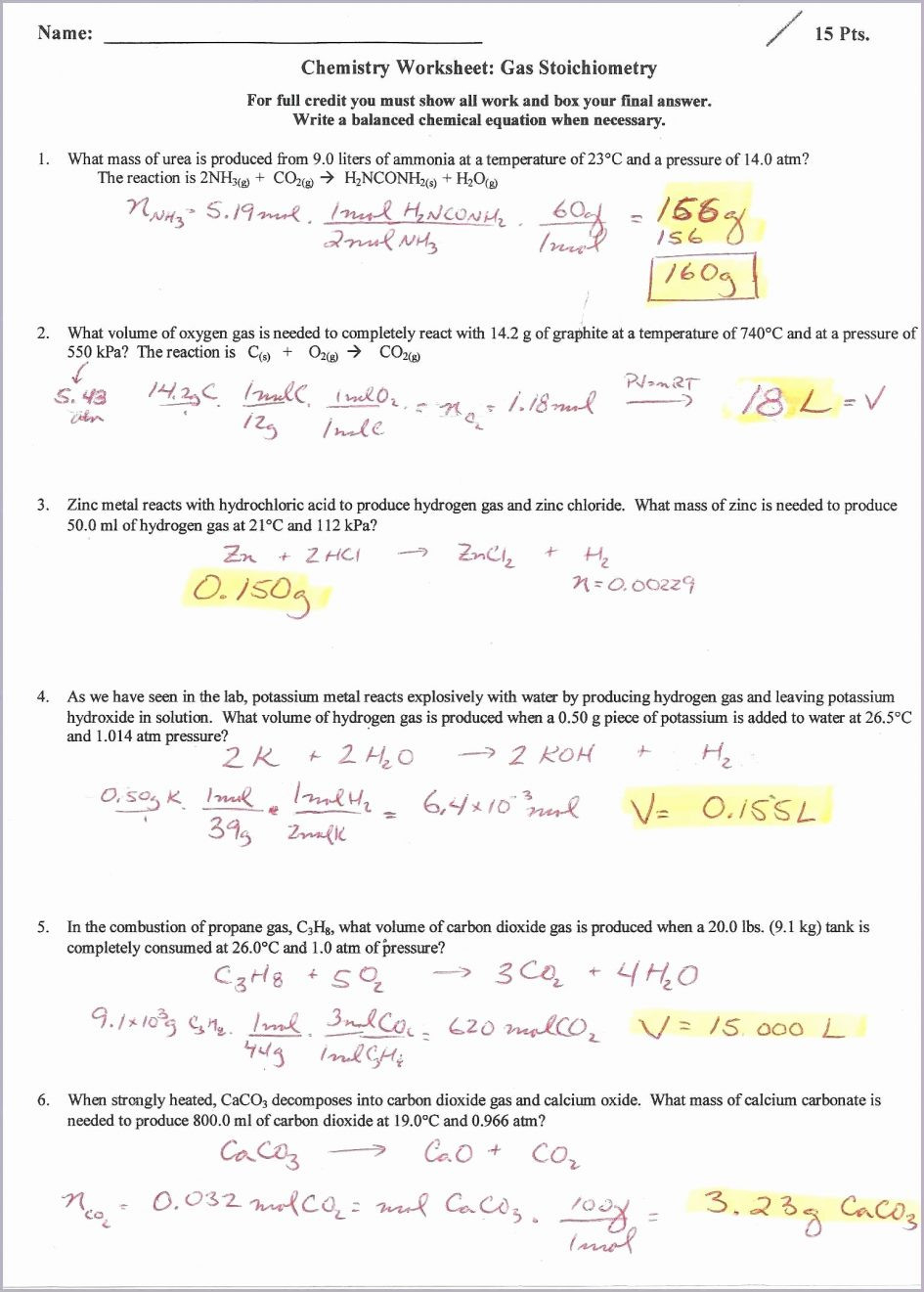
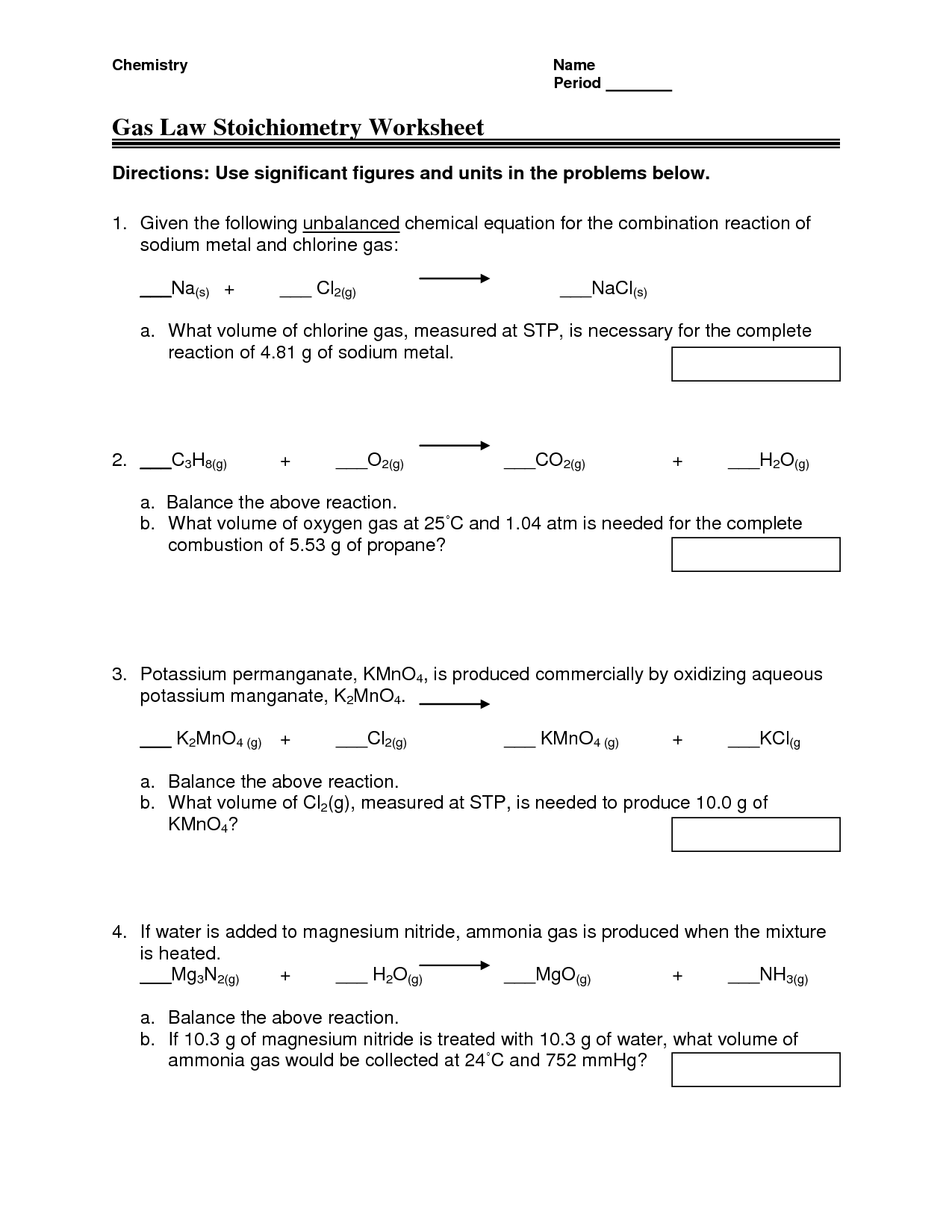
Ideal Gas Law And Stoichiometry Worksheet Answers
Ideal Gas Law And Stoichiometry Worksheet Answers - Web quiz & worksheet goals. Web why do you have to use the ideal gas law to solve this problem? Use the gas laws we have learned to solve each. Web the ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the ideal gas constant, and t is. The relevant formulas for calculations are:. Web ammonia and gaseous hydrogen chloride combine to form ammonium chloride according to this equation: Web gas law stoichiometry worksheet. Can't we take the number of grams in 1.50 l, divide it by 15.99*2 to get the number of moles, and then multiply it by. State what the 'n' represents in the ideal gas equation. For any sample of gas under ideal conditions, the relationship between the amount of gas in moles (n) and its temperature, pressure, and volume is. Identify an ideal gas condition. The relevant formulas for calculations are:. Web worksheet gas stoichiometry worksheet ideal gas law — db excel within ideal gas law worksheet image below, is part of ideal gas law worksheet. Use the gas laws we have learned to solve each. Web ideal gas law & gas stoichiometry worksheet. Can't we take the number of grams in 1.50 l, divide it by 15.99*2 to get the number of moles, and then multiply it by. Relating reaction stoichiometry and the ideal gas law. Web why do you have to use the ideal gas law to solve this problem? Web the ideal gas law states that pv=nrt, where p is the. The relevant formulas for calculations are:. This product includes a note sheet to walk your students through the basics of the ideal gas law, define stp, calculate the molar volume of a gas at stp and sample. Gas stoichiometry and the ideal gas law. When you take this quiz, you'll need to be able to: Use the gas laws we. At what temperature will 0.0100 mole of argon gas have a. Gas stoichiometry and the ideal gas law. The relevant formulas for calculations are:. Relating reaction stoichiometry and the ideal gas law. Web quiz & worksheet goals. A sample of hydrogen gas has a volume of. When you take this quiz, you'll need to be able to: Web this product includes a note sheet to walk your students through the basics of the ideal gas law, define stp, calculate the molar volume of a gas at stp and sample problems. Web why do you have to use. When you take this quiz, you'll need to be able to: Web why do you have to use the ideal gas law to solve this problem? Gas stoichiometry and the ideal gas law. Web worksheet gas stoichiometry worksheet ideal gas law — db excel within ideal gas law worksheet image below, is part of ideal gas law worksheet. The relevant. (convert your temperature to °k and use the right r value!!!) easy. Web ideal gas law & gas stoichiometry worksheet. What volume of carbon dioxide is produced by the combustion of 25.21 g of ethanol (c 2 h 5 oh) at. 1) for the reaction 2 h2(g) + o2(g) ( 2 h2o(g), how many liters of water can be made. At what temperature will 0.0100 mole of argon gas have a. Web why do you have to use the ideal gas law to solve this problem? State what the 'n' represents in the ideal gas equation. Can't we take the number of grams in 1.50 l, divide it by 15.99*2 to get the number of moles, and then multiply it. How many grams of alcl3 must decompose in order to produce 3.10 dm3 of cl2 at 50.0(c and 98.4 kpa? Relating reaction stoichiometry and the ideal gas law. The relevant formulas for calculations are:. You must correct to stp.) 2alcl3 ( 2al + 3cl2. Web ammonia and gaseous hydrogen chloride combine to form ammonium chloride according to this equation: 1) for the reaction 2 h2(g) + o2(g) ( 2 h2o(g), how many liters of water can be made from 5 l of oxygen gas and an excess of hydrogen at. Web why do you have to use the ideal gas law to solve this problem? Web this product includes a note sheet to walk your students through the basics. Web ideal gas law. Web gas law stoichiometry worksheet. Nh3(g) + hcl(g) → nh4cl(s) if 4.21 l of nh3(g) at 27°c and 1.02 atm is. The relevant formulas for calculations are:. Use the gas laws we have learned to solve each. Web ammonia and gaseous hydrogen chloride combine to form ammonium chloride according to this equation: You must correct to stp.) 2alcl3 ( 2al + 3cl2. At what temperature will 0.0100 mole of argon gas have a. When you take this quiz, you'll need to be able to: Web the ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the ideal gas constant, and t is. Relating reaction stoichiometry and the ideal gas law. Web quiz & worksheet goals. State what the 'n' represents in the ideal gas equation. What volume of carbon dioxide is produced by the combustion of 25.21 g of ethanol (c 2 h 5 oh) at. Identify an ideal gas condition. For any sample of gas under ideal conditions, the relationship between the amount of gas in moles (n) and its temperature, pressure, and volume is. This product includes a note sheet to walk your students through the basics of the ideal gas law, define stp, calculate the molar volume of a gas at stp and sample. (convert your temperature to °k and use the right r value!!!) easy. Web ideal gas law & gas stoichiometry worksheet. Gas stoichiometry and the ideal gas law. Web why do you have to use the ideal gas law to solve this problem? Web the ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the ideal gas constant, and t is. For any sample of gas under ideal conditions, the relationship between the amount of gas in moles (n) and its temperature, pressure, and volume is. When you take this quiz, you'll need to be able to: Web this product includes a note sheet to walk your students through the basics of the ideal gas law, define stp, calculate the molar volume of a gas at stp and sample problems. What volume of carbon dioxide is produced by the combustion of 25.21 g of ethanol (c 2 h 5 oh) at. Web gas law stoichiometry worksheet. Nh3(g) + hcl(g) → nh4cl(s) if 4.21 l of nh3(g) at 27°c and 1.02 atm is. Web worksheet gas stoichiometry worksheet ideal gas law — db excel within ideal gas law worksheet image below, is part of ideal gas law worksheet. Ideal gas stoichiometry worksheet directions: The relevant formulas for calculations are:. You must correct to stp.) 2alcl3 ( 2al + 3cl2. Can't we take the number of grams in 1.50 l, divide it by 15.99*2 to get the number of moles, and then multiply it by. State what the 'n' represents in the ideal gas equation. Web quiz & worksheet goals. Use the gas laws we have learned to solve each.13 Best Images of Pressure Problems Worksheet Answer Key
16 Pressure Problems Worksheet Answer Key /
18 Gas Law Calculations Worksheets Answers /
boyle's law worksheet key
11 Chemistry Gas Laws Worksheet /
Stoichiometry Worksheet Answer Key —
11 Chemistry Gas Laws Worksheet /
11 Chemistry Gas Laws Worksheet /
Ideal Gas Law Worksheets With Answers
Gas Stoichiometry Worksheet Answer Key
Relating Reaction Stoichiometry And The Ideal Gas Law.
A Sample Of Hydrogen Gas Has A Volume Of.
(Convert Your Temperature To °K And Use The Right R Value!!!) Easy.
Identify An Ideal Gas Condition.
Related Post: