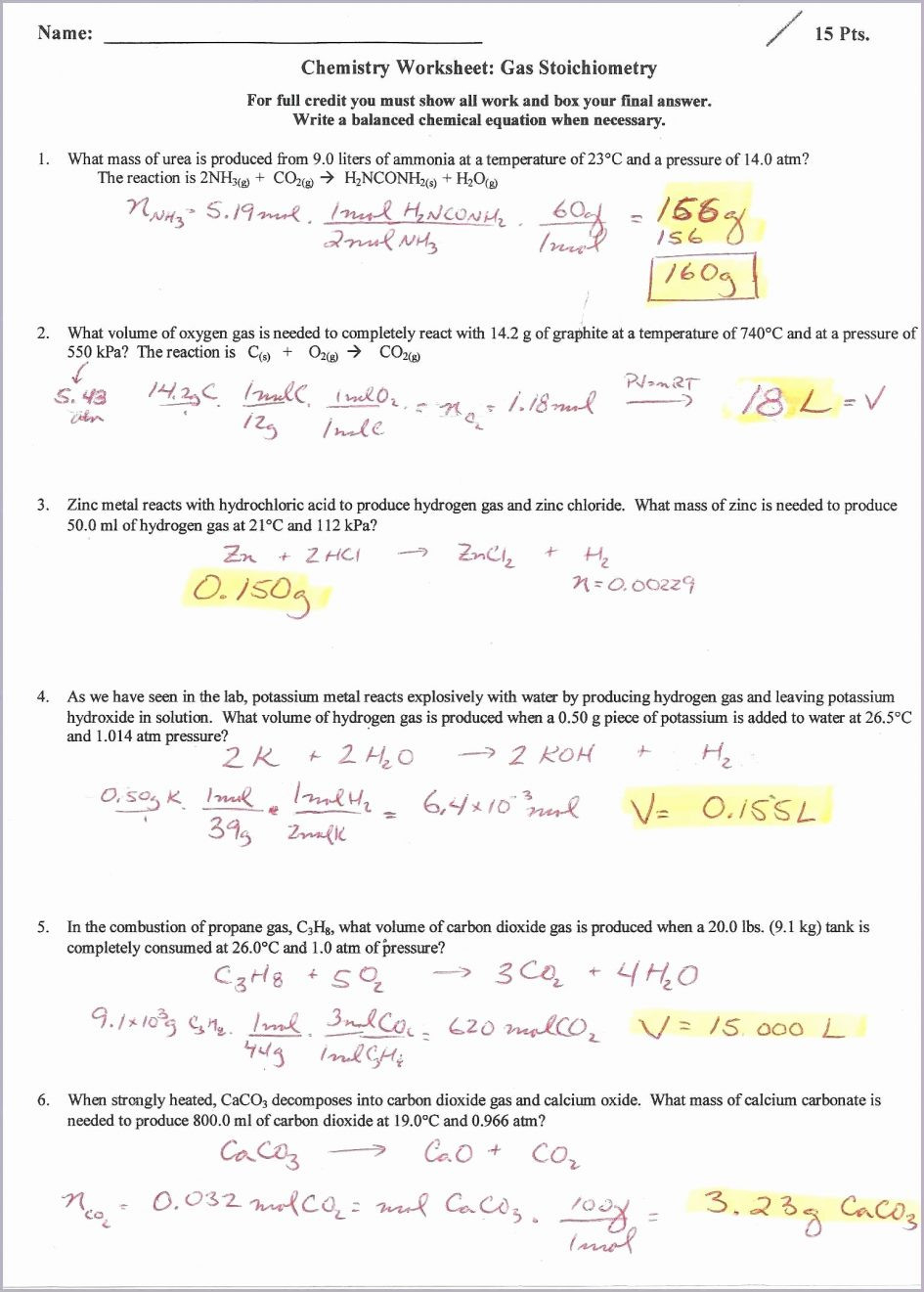
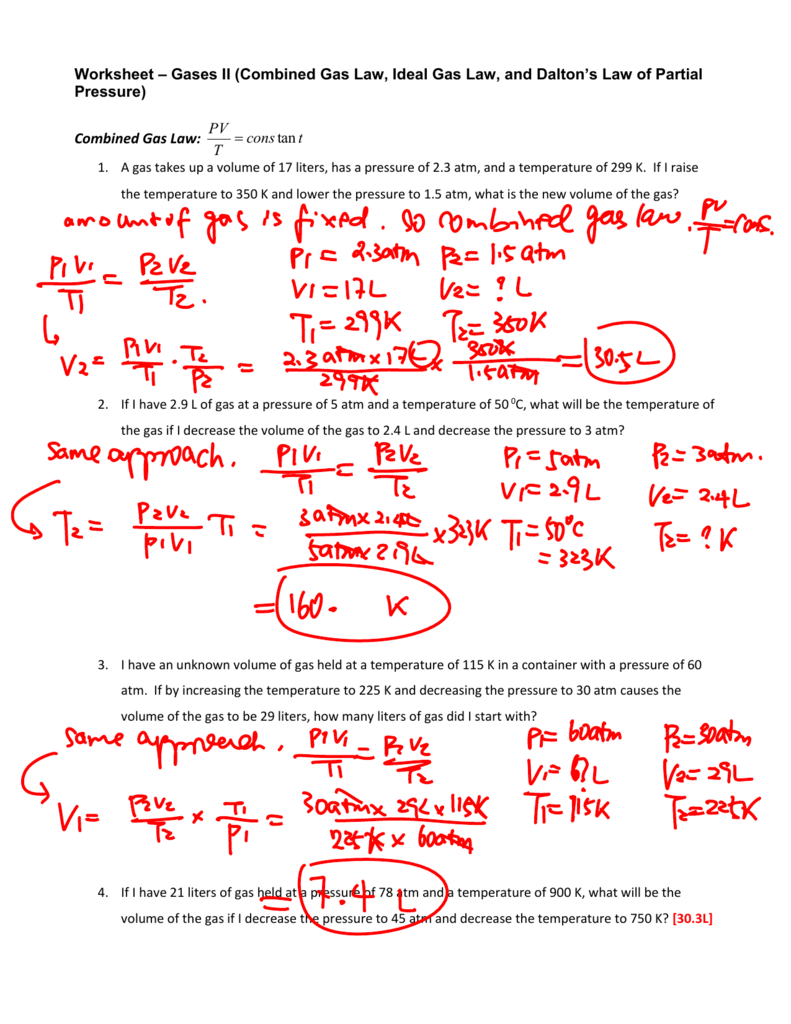
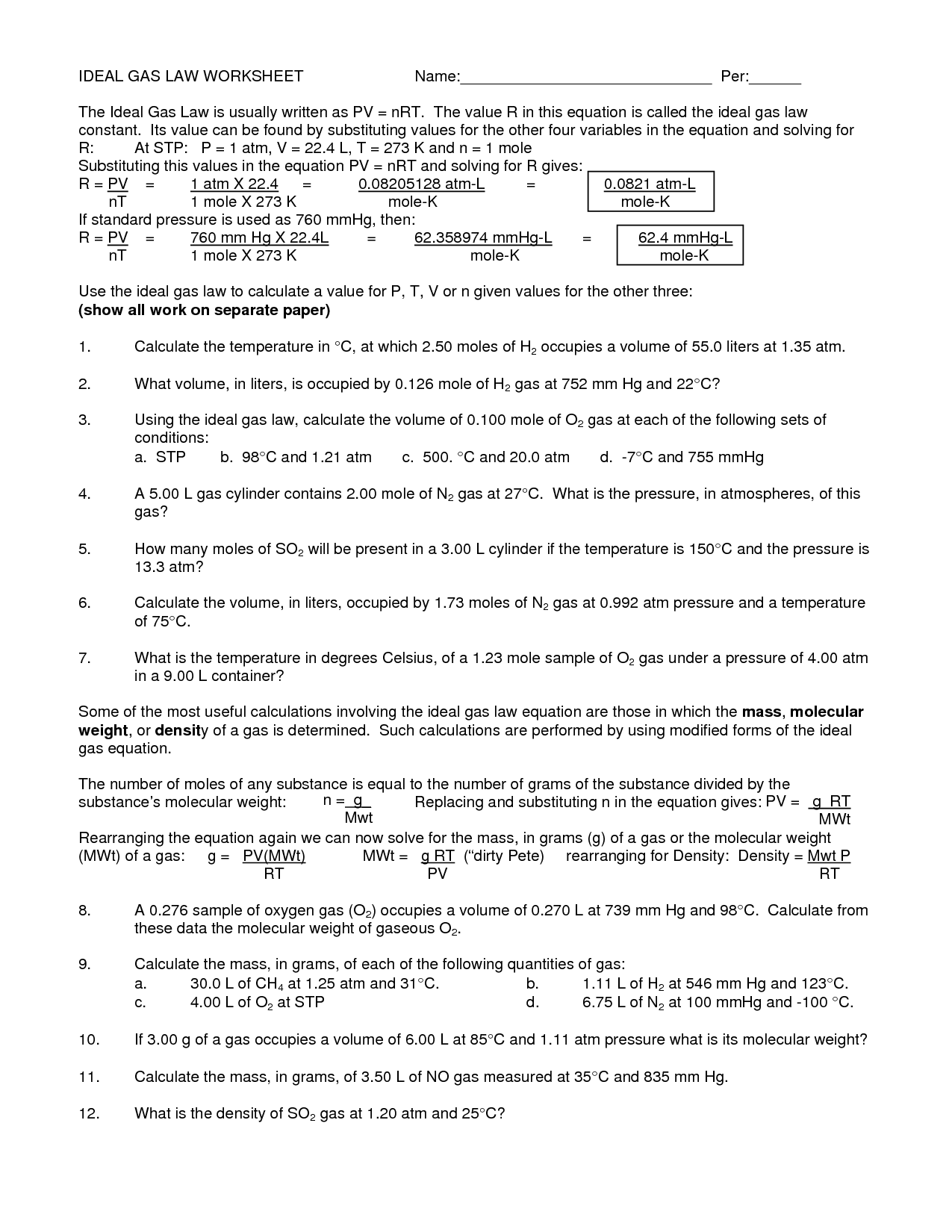
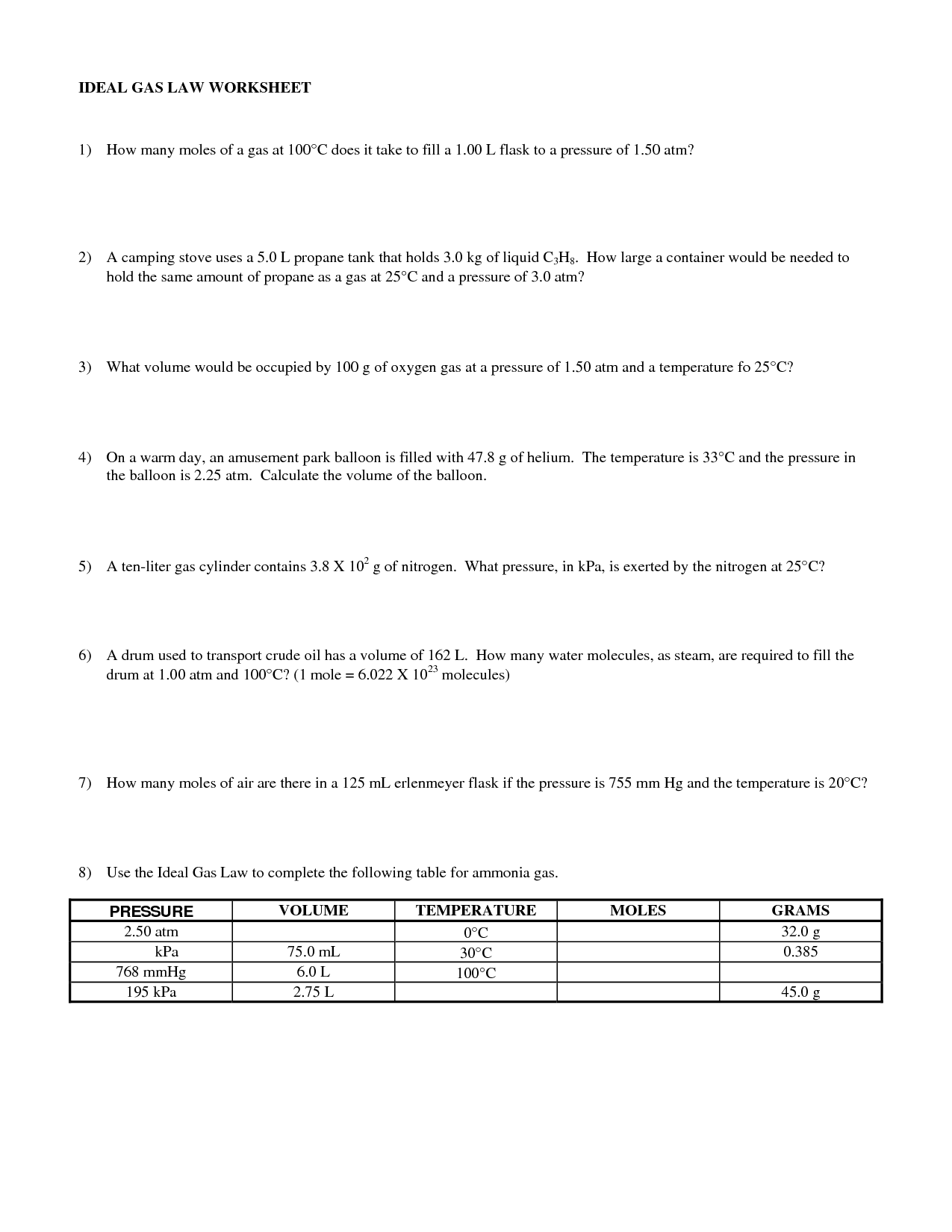
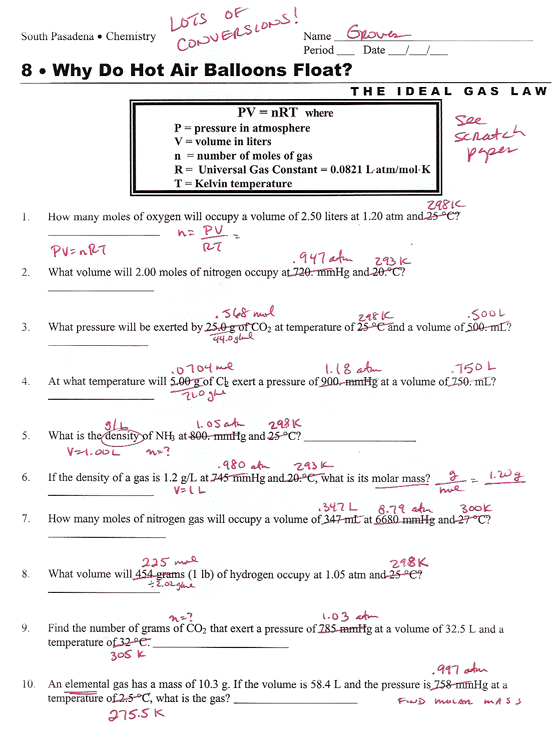
Ideal Gas Law Worksheet With Answers
Ideal Gas Law Worksheet With Answers - A) p = 1.01 atm v = ? Web ideal gas law practice worksheet solve the following problems using the ideal gas law: The ideal gas law investigates the relationship between pressure, volume, temperature, and moles of a gas. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Solve each of the following problems. The gas constant, r, is equal to 0.0821 when. Pv = nrt model 1: Web write your answer on the line provided. Solutions to the ideal gas law practice worksheet: A gas has a pressure of 799.0 mm hg at 50.0 °c. A) p = 1.01 atm v = ? The gas constant, r, is equal to 0.0821 when. If a gas is cooled from 343.0 k. Web to solve the following problems: Solutions to the ideal gas law practice worksheet: Web solutions to the ideal gas law practice worksheet: K*mol if pressure is needed in kpa then convert by multiplying by 101.3kpa / 1atm to get r =8.31 l*kpa / (k*mole) 1) if i have 4 moles of a. Solutions to the ideal gas law practice worksheet: The ideal gas law name: Part of the activity asks students to explain. This shows the relationship between a gas’s pressure ( p ), temperature ( t ), volume ( v ), and amount in moles ( n ). What is the temperature at standard pressure? The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is. On this worksheet. Web pv = constant charles’ law (1787) at a given pressure and number of moles of gas, charles' law states that the volume and temperature of a gas are directly proportional. Web what is the pressure at standard temperature? A gas has a pressure of 799.0 mm hg at 50.0 °c. N = 0.00831 mol t = 25°c b) p. Web write your answer on the line provided. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Web ideal gas law name ___________ 1) given the following sets of values, calculate the unknown quantity. The ideal gas law states that pv=nrt, where p is the pressure of a gas,. Web the ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is the number of moles of gas present, r is the ideal gas constant, and t is. The gas constant, r, is equal to 0.0821 when. This activity should be done before the ideal gas law. The gas constant, r, is equal to 0.0821 when. Web the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the. Assume that the lungs are at 1.00 atm pressure and at a body temperature of 40 oc. Web ideal gas law practice worksheet. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is. What is the temperature at standard pressure? Web write your answer on the line provided. Web the ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of. This activity should be done before the ideal gas law is presented in class. Web solutions to the ideal gas law practice worksheet: Web the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the. Web ideal gas law name ___________ 1) given the. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is. Pv = nrt model 1: The ideal gas law investigates the relationship between pressure, volume, temperature, and moles of a gas. Real gases behave like ideal gases except at very high temperatures. Solutions to the ideal. 1) how many moles of gas does it take to occupy 120 liters at a pressure of 2.3. Pv = nrt model 1: Part of the activity asks students to explain how volume, temperature, and number of moles affect. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Web to solve the following problems: Solutions to the ideal gas law practice worksheet: On this worksheet you will practice with the ideal gas law, the combined gas law, as well as the relationships between the number of moles, the mass, and the. Web the ideal gas law describes the behavior of an ideal gas, a hypothetical substance whose behavior can be explained quantitatively by the ideal gas law and the. The ideal gas law investigates the relationship between pressure, volume, temperature, and moles of a gas. Web the observed behavior of gases, embodied in the empirical gas laws, leads to a series of equations that can be summarized by a single equation of state, called the ideal gas law equation. Web write your answer on the line provided. Web solutions to the ideal gas law practice worksheet: Real gases behave like ideal gases except at very high temperatures. N = 0.00831 mol t = 25°c b) p = ? Web pv = constant charles’ law (1787) at a given pressure and number of moles of gas, charles' law states that the volume and temperature of a gas are directly proportional. Web write your answer on the line provided. This activity investigates the relationship between pressure, volume, temperature, and number of moles for an ideal gas. A) p = 1.01 atm v = ? The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is. Assume that the lungs are at 1.00 atm pressure and at a body temperature of 40 oc. The ideal gas law name: This activity should be done before the ideal gas law is presented in class. Solutions to the ideal gas law practice worksheet: What is the temperature at standard pressure? Web write your answer on the line provided. T(k) is always ≥ 0 k • boyle’s law. The ideal gas law states that pv=nrt, where p is the pressure of a gas, v is the volume of the gas, n is. N = 0.00831 mol t = 25°c b) p = ? A gas has a pressure of 799.0 mm hg at 50.0 °c. Web pv = constant charles’ law (1787) at a given pressure and number of moles of gas, charles' law states that the volume and temperature of a gas are directly proportional. This activity investigates the relationship between pressure, volume, temperature, and number of moles for an ideal gas. Web write your answer on the line provided. This shows the relationship between a gas’s pressure ( p ), temperature ( t ), volume ( v ), and amount in moles ( n ). Web what is the pressure at standard temperature? The ideal gas law investigates the relationship between pressure, volume, temperature, and moles of a gas. Part of the activity asks students to explain how volume, temperature, and number of moles affect.13 Best Images of Pressure Problems Worksheet Answer Key
boyle's law worksheet key
Worksheet K4 Chemistry Dalton's Law Law Worksheet
18 Gas Law Calculations Worksheets Answers /
15 Best Images of Ideal Gas Law Worksheet Ideal Gas Law Worksheet
Avogadro's Law Worksheet Answers / Solved Experiment 14 Using The Ideal
Ideal Gas Law Gizmo Answer Key Pdf
Ideal Gas Law Worksheet 144 Answer Key Greenus
Ideal Gas Law Worksheet 144 Answer Key Greenus
Ideal Gas Law Worksheet
On This Worksheet You Will Practice With The Ideal Gas Law, The Combined Gas Law, As Well As The Relationships Between The Number Of Moles, The Mass, And The.
Real Gases Behave Like Ideal Gases Except At Very High Temperatures.
Web The Ideal Gas Law States That Pv=Nrt, Where P Is The Pressure Of A Gas, V Is The Volume Of The Gas, N Is The Number Of Moles Of Gas Present, R Is The Ideal Gas Constant, And T Is.
How Many Moles Of Gas (Air) Are In The Lungs Of An Adult With A Lung Capacity Of 3.9 L?
Related Post: