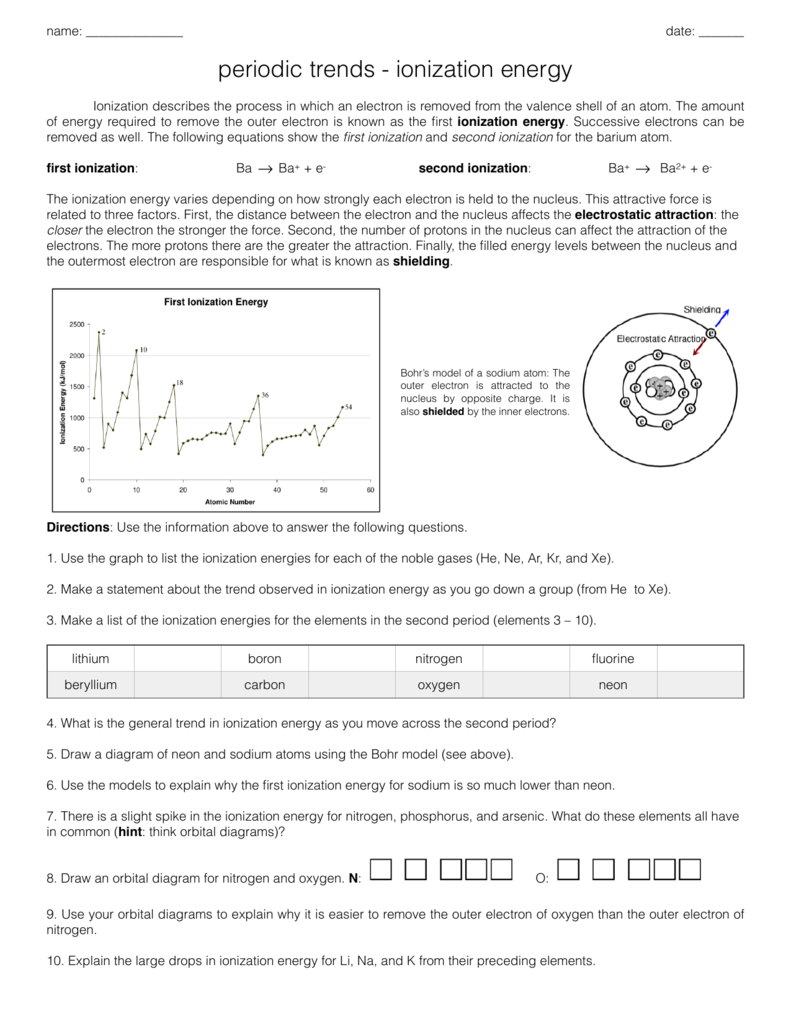
Ionization Energy Worksheet
Ionization Energy Worksheet - Web key for coulomb’s law/ionization energy worksheet coulomb’s law coulomb’slawstatesthattheforcebetweentwoparticlesisdirectlyproportionaltotheproductoftheir individualcharges,q,andinverselypropotionaltothesquareofthedistance,d,thatseparatesthem f∝ q 1×q 2 d2 theproductofforceanddistancegivespotentialenergy,e;thus e∝ q 1×q 2 d Web ionization energy worksheet view preview ; Some of the worksheets for this concept are bonding basics, bondingbasics2008, ionic and covalent compounds name key, , chapter 7 practice work covalent bonds and molecular, types of chemical bonds key, work chemical bonding ionic covalent, naming ionic compounds. If so, describe the trend. Some of the worksheets for this concept are key for coulombs lawionization energy work, work 12, key for first ionization energy work, work 11, aim determining ionization energy and electronegativity, periodic trends work, multiple ionization energies, chapter 8 practice. First ionization energy, ie(1), shown in purple square. Web the first ionization energy, \(i_1\), refers to removing one electron from a neutral atom: Which element has the lowest ionization energy? Describing the factors affecting ionisation energy; The ænount ot energy to remove the outer electron is known as the first. Some of the worksheets for this concept are key for coulombs lawionization energy work, work 12, key for first ionization energy work, work 11, aim determining ionization energy and electronegativity, periodic trends work, multiple ionization energies, chapter 8 practice. The amount of energy required to remove the outer electron is called the first ionization energy. Can a periodic trend be. Here are some of last week's most popular sheets in the meantime. Which group has the lowest? The amount of energy required to remove the outer electron is called the first ionization energy. Which element has the highest ionization energy? Which group (or chemical family) has the highest ionization energies? Third ionization energy, ie(3), shown in pink triangle. Web what is ionization energy? The amount of energy required to remove the outer electron is called the first ionization energy. The following equations show the first and second ionization for the nitrogen atom. This can be determined by its position lowest in the alkali metal group. Wh words by shaleeza all about plants by shaleeza مذکر مونث by quratulain103 homophones by We're sorry, but there were no search results for ionization energy. Worksheets are key for coulombs lawionization energy work, work 12, key for first ionization energy work, work 11, aim determining ionization energy and electronegativity, periodic trends work, multiple ionization energies, chapter 8 practice work.. The ænount ot energy to remove the outer electron is known as the first. Q.11 why do first ionisation energies decrease down a group? Successive electrons can be removed as well. Web ionization energy worksheet view preview ; This can be determined by its position lowest in the alkali metal group. The energy needed to remove an electron from an atom. Add to my workbooks (2) download file pdf embed in my website or blog add to google classroom add to microsoft teams share through whatsapp link to this worksheet: Watch videos and use nagwa’s tools and apps to help students achieve their full potential. Q.11 why do first ionisation energies. Web explore and practice nagwa’s free online educational courses and lessons for math and physics across different grades available in english for egypt. Third ionization energy, ie(3), shown in pink triangle. Can a periodic trend be observed? Web worksheets exploring the concept of ionization energy, factors affecting ionization energy and periodic trends in ionization energy.questions cover:+ definition of (first) ionization. Web worksheets exploring the concept of ionisation energy, factors affecting ionisation energy and periodic trends in ionisation energy. Second ionization energy, ie(2), shown in green circle. This can be determined by its position lowest in the alkali metal group. Web ionization energy worksheets search results: Representing ionisation energies with equations; *click on open button to open and print. Second ionization energy, ie(2), shown in green circle. Third ionization energy, ie(3), shown in pink triangle. Lesson menu lesson lesson worksheet Web ionization energy worksheets search results: Successive electrons can be removed as well. *click on open button to open and print. Here are some of last week's most popular sheets in the meantime. Web what is ionization energy? Web explore and practice nagwa’s free online educational courses and lessons for math and physics across different grades available in english for egypt. The ænount ot energy to remove the outer electron is known as the first. Describing the factors affecting ionisation energy; If so, describe the trend. Here are some of last week's most popular sheets in the meantime. Examine the graph of atomic radius vs atomic number. Web explore and practice nagwa’s free online educational courses and lessons for math and physics across different grades available in english for egypt. The following equations show the first and second ionization for the nitrogen atom. Rank the following in order from lowest to highest ionization energy? Write the charge that each of the following atoms will have when it has a complete set of valence electrons forming an ion. Web the first ionization energy is for the electron in then= 2shell because it is furthest from the nucleus;we call electrons in the outermost shell valence electrons and we identify all other electrons as core electrons. Web worksheets exploring the concept of ionization energy, factors affecting ionization energy and periodic trends in ionization energy.questions cover:+ definition of (first) ionization energies+ representing ionization energies with equations+ describing the factors affecting ionization energy + demonstrating understanding of successive. Compare the values of ie1 for elements in the second row to the elements directly below them in the third row? The amount of energy required to remove the outer electron is called the first ionization energy. Data from inorganic chemistry by shriver and atkins. This can be determined by its position lowest in the alkali metal group. Which element has the lowest ionization energy? Web worksheets exploring the concept of ionisation energy, factors affecting ionisation energy and periodic trends in ionisation energy. Successive electrons can be removed as well. Web key for coulomb’s law/ionization energy worksheet coulomb’s law coulomb’slawstatesthattheforcebetweentwoparticlesisdirectlyproportionaltotheproductoftheir individualcharges,q,andinverselypropotionaltothesquareofthedistance,d,thatseparatesthem f∝ q 1×q 2 d2 theproductofforceanddistancegivespotentialenergy,e;thus e∝ q 1×q 2 d *click on open button to open and print. Third ionization energy, ie(3), shown in pink triangle. Definition of (first) ionisation energies; The ænount ot energy to remove the outer electron is known as the first. Compare the values of ie1 for elements in the second row to the elements directly below them in the third row? K < na < li. Some of the worksheets for this concept are bonding basics, bondingbasics2008, ionic and covalent compounds name key, , chapter 7 practice work covalent bonds and molecular, types of chemical bonds key, work chemical bonding ionic covalent, naming ionic compounds. Web explore and practice nagwa’s free online educational courses and lessons for math and physics across different grades available in english for egypt. Rank the following in order from lowest to highest ionization energy? This can be determined by its position lowest in the alkali metal group. Successive electrons can be removed as well. Web the first, second, and third ionization energies for the first four rows of the periodic table. Which element has the lowest ionization energy? We're sorry, but there were no search results for ionization energy. Q.11 why do first ionisation energies decrease down a group? Web ionization energy worksheet view preview ; Web ionization describes the process in which an electron is removed from an atom in the gaseous state.Worksheet Ionization Energy
ionization energy worksheet with answers
Chemistry Ionization Energies Worksheets
Successive ionization energies worksheet
First Ionization Energy Trend Graph Images Amashusho
Quiz & Worksheet Ionization Energy
Electron Configurations and Ionization Energy Worksheet for 9th 12th
Practice Ionization Energy & Orbitals Worksheet printable pdf download
️Chemistry Ionization Energies Worksheet Free Download Goodimg.co
5 Ionisation Energies
Web Key For Coulomb’s Law/Ionization Energy Worksheet Coulomb’s Law Coulomb’slawstatesthattheforcebetweentwoparticlesisdirectlyproportionaltotheproductoftheir Individualcharges,Q,Andinverselypropotionaltothesquareofthedistance,D,Thatseparatesthem F∝ Q 1×Q 2 D2 Theproductofforceanddistancegivespotentialenergy,E;Thus E∝ Q 1×Q 2 D
Which Element Has The Highest Ionization Energy?
Web The First Ionization Energy Is For The Electron In Then= 2Shell Because It Is Furthest From The Nucleus;We Call Electrons In The Outermost Shell Valence Electrons And We Identify All Other Electrons As Core Electrons.
Some Of The Worksheets For This Concept Are Key For Coulombs Lawionization Energy Work, Work 12, Key For First Ionization Energy Work, Work 11, Aim Determining Ionization Energy And Electronegativity, Periodic Trends Work, Multiple Ionization Energies, Chapter 8 Practice.
Related Post: