Limiting Reagent And Percent Yield Worksheet
Limiting Reagent And Percent Yield Worksheet - Web limiting reagent and percent yield worksheet take the reaction: \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\% percent yield = theoretical yieldactual yield ×. Web use this information to answer the following questions a) write a balance the equation for this double replacement reaction: Web limiting reagent and percent yield worksheet name period 1. Practice the calculations to find the limiting reagents and yields. This worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent. The limiting reagent gives the smallest yield of product calculated from the reagents. Web percent yield problem #1 • what is the percent yield of this reaction if 24.8 g of caco 3 is heated to give 13.1 g of cao? Web limiting reactant and percentage yield. Limiting reagent worksheet #2 author: Caco 3 cao + co 2 1. Web limiting reagent and percent yield worksheet take the reaction: Limiting reagent worksheet #2 author: Web the percent yield is calculated as follows: This reagent is the one that determines the amount of product formed. Web use this information to answer the following questions a) write a balance the equation for this double replacement reaction: Web a limiting reagent is a chemical reactant that limits the amount of product that is formed. Web limiting reactant and percentage yield. Web limiting reagent and percent yield worksheet take the reaction: Web the percent yield is calculated as. This reagent is the one that determines the amount of product formed. Web limiting reagent and percent yield worksheet name period 1. Caco 3 cao + co 2 1. Web what is the percent yield of a reaction in which 41.5 g of solid tungsten(vi) oxide reacts with. Web 4) how much of the nonlimiting reagent is left over in. Answers to worksheet #14 limiting reagents a limiting reagent is the reactant that is completely used up in a reaction. Limiting reagent calculations are performed This reagent is the one that determines the amount of product formed. Web limiting reagent and percent yield worksheet name period 1. Web that said, the coefficients of the balanced equation have nothing to do. Practice the calculations to find the limiting reagents and yields. Web limiting reagent and percent yield worksheet take the reaction: Web what is the percent yield of a reaction in which 41.5 g of solid tungsten(vi) oxide reacts with. In an experiment, 3.25 g of nh3 are allowed to react with 3.50 g of o2. When copper (ii) chloride reacts. Web a limiting reagent is a chemical reactant that limits the amount of product that is formed. Web percent yield problem #1 • what is the percent yield of this reaction if 24.8 g of caco 3 is heated to give 13.1 g of cao? Caco 3 cao + co 2 1. Limiting reagent calculations are performed Cucl2 + 2. Caco 3 cao + co 2 1. Web understanding limiting and excess reagents. The limiting reagent gives the smallest yield of product calculated from the reagents. Web that said, the coefficients of the balanced equation have nothing to do with the actual quantity of reactants you start with, as you can mix any amount you choose, but clearly. In an. Web limiting reagent and percent yield worksheet take the reaction: Nh3 + o2 no + h2o. Answers to worksheet #14 limiting reagents a limiting reagent is the reactant that is completely used up in a reaction. Limiting reagent worksheet #2 author: This reagent is the one that determines the amount of product formed. Predict quantities of products produced or reactants consumed based on complete consumption of limiting reagent (on both mole. Answers to worksheet #14 limiting reagents a limiting reagent is the reactant that is completely used up in a reaction. Web 4) how much of the nonlimiting reagent is left over in this reaction? When copper (ii) chloride reacts with sodium nitrate,. Limiting reagent worksheet #2 author: The limiting reagent gives the smallest yield of product calculated from the reagents. Web that said, the coefficients of the balanced equation have nothing to do with the actual quantity of reactants you start with, as you can mix any amount you choose, but clearly. In an experiment, 3.25 g of nh3 are allowed to. This reagent is the one that determines the amount of product formed. Web limiting reagent and percent yield worksheet take the reaction: Nh3 + o2 no + h2o. Predict quantities of products produced or reactants consumed based on complete consumption of limiting reagent (on both mole. \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\% percent yield = theoretical yieldactual yield ×. Web percent yield problem #1 • what is the percent yield of this reaction if 24.8 g of caco 3 is heated to give 13.1 g of cao? Web what is the percent yield of a reaction in which 41.5 g of solid tungsten(vi) oxide reacts with. The limiting reagent gives the smallest yield of product calculated from the reagents. Limiting reagent worksheet #2 author: Web a limiting reagent is a chemical reactant that limits the amount of product that is formed. Caco 3 cao + co 2 1. Web after learning how to solve stoichiometric problems, this is an introduction to the application of that process for both determining the limiting reagent and percent yield. Practice the calculations to find the limiting reagents and yields. Web the percent yield is calculated as follows: Web limiting reagent and percent yield. Web limiting reactant and percentage yield. Web limiting reagent and percent yield worksheet name period 1. Web up to $3 cash back limiting reagents and percentage yield worksheet 1. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Web up to 24% cash back limiting reagents and percentage yield worksheet 1. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Web the percent yield is calculated as follows: \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\% percent yield = theoretical yieldactual yield ×. Predict quantities of products produced or reactants consumed based on complete consumption of limiting reagent (on both mole. Web a limiting reagent is a chemical reactant that limits the amount of product that is formed. This reagent is the one that determines the amount of product formed. Web limiting reactant and percentage yield. Practice the calculations to find the limiting reagents and yields. In an experiment, 3.25 g of nh3 are allowed to react with 3.50 g of o2. Cucl2 + 2 nano3 ( cu(no3)2 + 2 nacl. Web up to $3 cash back limiting reagents and percentage yield worksheet 1. Web understanding limiting and excess reagents. Web 4) how much of the nonlimiting reagent is left over in this reaction? Web what is the percent yield of a reaction in which 41.5 g of solid tungsten(vi) oxide reacts with. Limiting reagent calculations are performed Web after learning how to solve stoichiometric problems, this is an introduction to the application of that process for both determining the limiting reagent and percent yield.Otis Worksheet List Of Limiting Reactant And Percent Yield Worksheet
limiting reagent and percent yield worksheet
Limiting Reagents and Percentage Yield Worksheet Zinc Toluene
Limiting Reagents Percent Yield Worksheet Stoichiometry Nitrogen
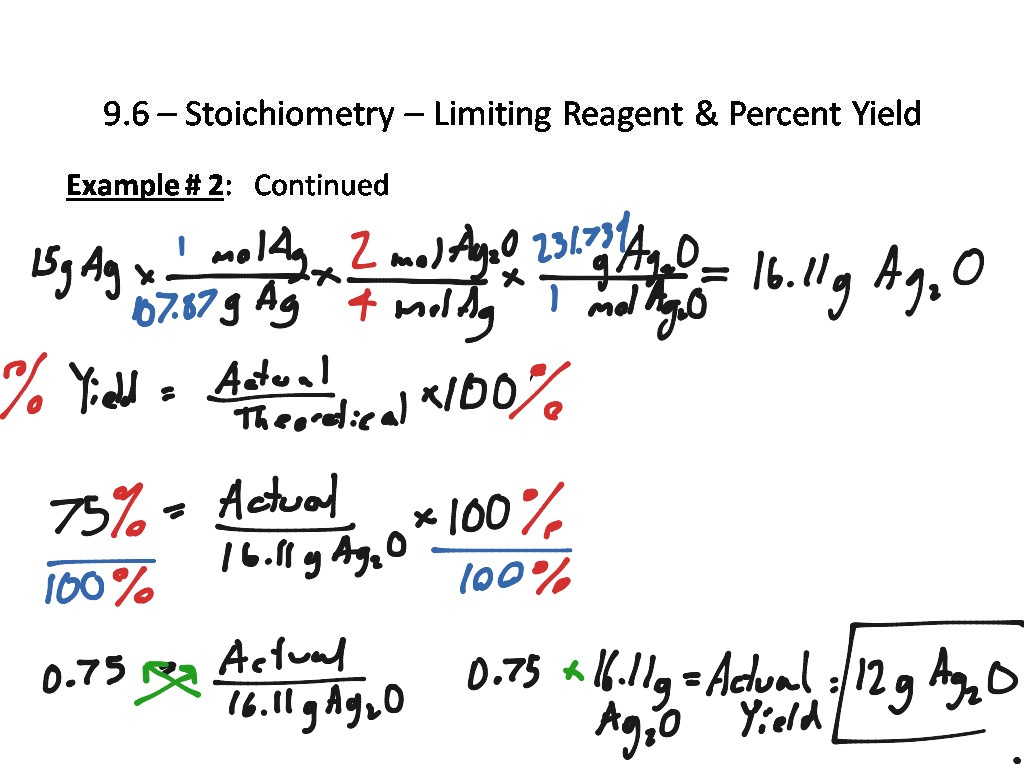
Stoichiometry, Percent Yield and Limiting Reagents practice problems
Stoichiometry Limiting Reagent Worksheet Photos Cantik
Limiting Reactant and percent yield worksheets
Limiting Reagent Worksheet 2
Quiz & Worksheet Calculating Reaction Yield and Percentage Yield from
Limiting Reagents and Percentage Yield Worksheet Answers PDF Mole
Web Limiting Reagent And Percent Yield.
Web Up To 24% Cash Back Limiting Reagents And Percentage Yield Worksheet 1.
Nh3 + O2 No + H2O.
Caco 3 Cao + Co 2 1.
Related Post: