Molarity And Molality Worksheet
Molarity And Molality Worksheet - Molality = number of moles of. Change solutes to compare different chemical compounds in. Web molality is independent of temperature so it is used for calculating boiling point elevation and freezing point depression. A complete answer key is provided at the end. What is the molality of ions in solution if 2.0 moles of al(no3)3 is added to 2.5 kg of water? Web this worksheet provides many examples for students to practice calculations involving molarity & Web 260 + results sort by: Calculate grams of solute needed to. Web molarity molarity boiling point elevation and freezing point depression molarity calculations science > chemistry library > states of matter and intermolecular forces > molarity. Web thesolutions density is 0.90 g/ml.7. Web molarity molarity boiling point elevation and freezing point depression molarity calculations science > chemistry library > states of matter and intermolecular forces > molarity. Calculate grams of solute needed to. Web molarity and molality practice worksheet. What is the molality of bromide ions in solution? Molality = number of moles of. Molality = number of moles of. Mole fraction (x) and mole % moles of component / total. Web up to 24% cash back concentrations worksheet: A complete answer key is. Web molarity and molality practice worksheet. Web molarity / molality worksheet name: Molality = number of moles of. Web calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. What is the molality of bromide ions in solution? List molarity and molality practice worksheet created by mj this worksheet provides many examples for students to practice. Web molality is independent of temperature so it is used for calculating boiling point elevation and freezing point depression. Molality (m) moles of solute mass 1) 2) 5) 6) 7) 9) 10) molarity (m) moles of solute liters o solution define molarity. Calculate grams of solute needed to. Web thesolutions density is 0.90 g/ml.7. Molarity and molality concentration is a. Molarity, molality, and normality are all units of concentration in chemistry. Change solutes to compare different chemical compounds in. Web up to 24% cash back concentrations worksheet: Web you can also take the definition of molarity, m = mol/l, and the definition of molar mass, mm=g/mol. Molality (m) moles of solute mass 1) 2) 5) 6) 7) 9) 10) molarity. Web molarity / molality worksheet name: Web up to 6% cash back explanation: Web learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. Molarity is defined as the number of moles of solute per liter of. Molality = number of moles of. Web learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. What is the molality of ions in solution if 2.0 moles of al(no3)3 is added to 2.5 kg of water? Change solutes to compare different chemical compounds in. Web a solution of mgbr2 is 1.40 m. Web this worksheet provides many. Web calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. A complete answer key is. Web up to 24% cash back concentrations worksheet: Molarity is defined as the number of moles of solute per liter of. Molality (m) moles of solute mass 1) 2) 5) 6) 7) 9) 10) molarity (m) moles of solute liters o. Solve for mol in the latter (mol =g/mm) and plug it into the former to get m =. Web thesolutions density is 0.90 g/ml.7. Molality = number of moles of. Web this worksheet provides many examples for students to practice calculations involving molarity & Web calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Web molarity = moles of solute / liters of solution (abbreviation = m) molality = moles of solute / kg of solvent (abbreviation = m) normality = number of equivalent of solute x molarity of. Web up to 6% cash back explanation: What is the molality of bromide ions in solution? Molarity is defined as the number of moles of. Molality (m) moles of solute mass 1) 2) 5) 6) 7) 9) 10) molarity (m) moles of solute liters o solution define molarity. Molarity is defined as the number of moles of solute per liter of. What is the molality of bromide ions in solution? Web you can also take the definition of molarity, m = mol/l, and the definition of molar mass, mm=g/mol. Web thesolutions density is 0.90 g/ml.7. What is the molality of ions in solution if 2.0 moles of al(no3)3 is added to 2.5 kg of water? List molarity and molality practice worksheet created by mj this worksheet provides many examples for students to practice. Calculate grams of solute needed to. Web molarity and molality practice worksheet. A complete answer key is provided at the end. Solve for mol in the latter (mol =g/mm) and plug it into the former to get m =. Web up to 24% cash back concentrations worksheet: A complete answer key is. Web molarity molarity boiling point elevation and freezing point depression molarity calculations science > chemistry library > states of matter and intermolecular forces > molarity. Change solutes to compare different chemical compounds in. Molarity and molality concentration is a measurement of how much solute (substance) is in a given amount of solvent (liquid). A commonly purchased disinfectant is a 3.0% (by mass) solution of hydrogenperoxide (h 2 o 2 ) in water. Web calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. Web number of moles of solute (kcl) = given mass/ molecular mass. Molarity is defined as the number of moles of solute per liter of. Web thesolutions density is 0.90 g/ml.7. Web up to 24% cash back concentrations worksheet: A commonly purchased disinfectant is a 3.0% (by mass) solution of hydrogenperoxide (h 2 o 2 ) in water. Web number of moles of solute (kcl) = given mass/ molecular mass. Calculate grams of solute needed to. Web learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. Web molarity and molality practice worksheet. Solve for mol in the latter (mol =g/mm) and plug it into the former to get m =. Web a solution of mgbr2 is 1.40 m. Change solutes to compare different chemical compounds in. What is the molality of ions in solution if 2.0 moles of al(no3)3 is added to 2.5 kg of water? Web this worksheet provides many examples for students to practice calculations involving molarity & What is the molality of bromide ions in solution? A complete answer key is. Web molarity = moles of solute / liters of solution (abbreviation = m) molality = moles of solute / kg of solvent (abbreviation = m) normality = number of equivalent of solute x molarity of.Molarity Worksheet 1 worksheet
Molarity, Molality, Mass Percent, Mole Fraction, and Normality
Unit 9 Worksheet 3 Molar Concentration
7 Molarity Worksheet With Answers /
Conversion from Molality to Molarity
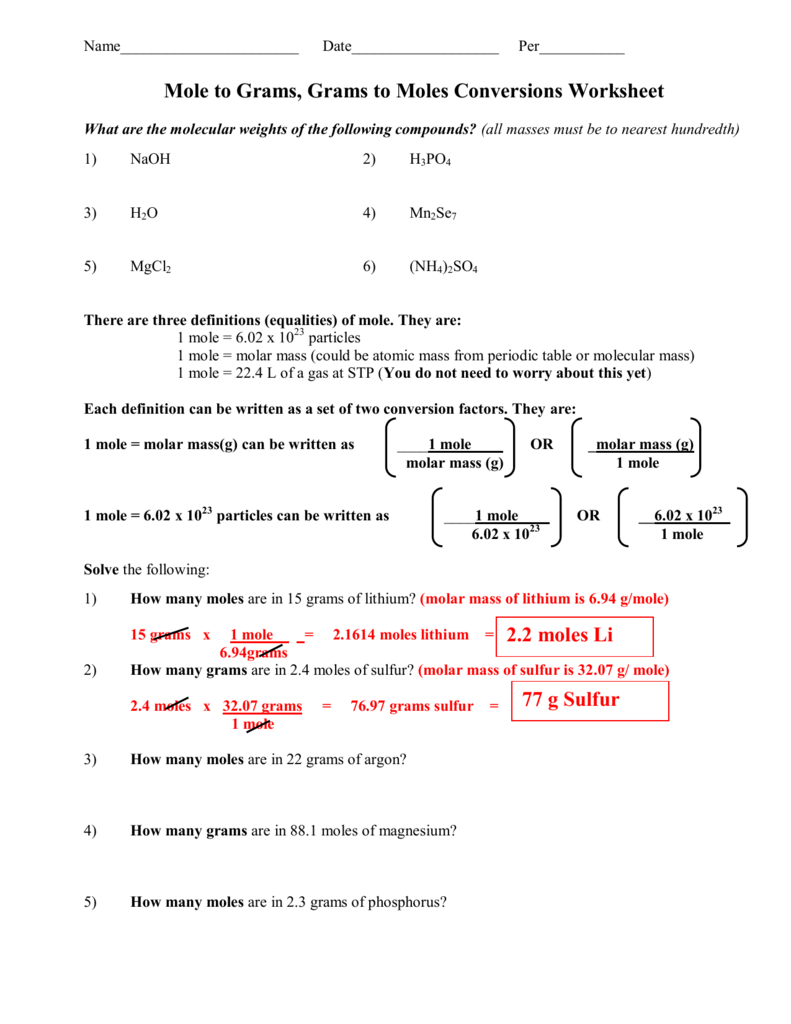
1 Mole Calculation Worksheet
7 Molarity Worksheet With Answers /
14 Chemistry Mole Practice Worksheet /
Molarity Practice Worksheet Answer
Molarity_Worksheet 1 ans key Molar Concentration Magnesium
In This Article, We'll Look At How To Describe.
Web Calculate Molarity If 25.0 Ml Of 1.75 M Hcl Diluted To 65.0 Ml.
Web Molarity / Molality Worksheet Name:
Mole Fraction (X) And Mole % Moles Of Component / Total.
Related Post: