Percent Yield Worksheet Answers
Percent Yield Worksheet Answers - Any yield under 100% is reasonable under the law of conservation of mass. Calculating the theoretical yield and the percent yield. Sn3(po4)4 + 6 na2co3 →. 1) balance the equation for the. 3 + 3 mg(oh) 2. Grams of sodium nitrate, how much sodium. 16.9% 2) a) if i start with 5 grams of c 3h8, what is my theoretical yield of water? This worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. Nh 4 no 3 + na 3 po 4 (nh 4 3 po 4 + nano 3 which reactant is limiting, assuming we started with 30.0 grams of ammonium. (98.639 + 99.042)/2 = 98.8 %. Yield is 17 g nh. All of the work is shown also.docx filethechemteacher.weebly.comthe chemistry teacher on. 3, % yield = 82.3%. 5) if i do this reaction with 15 grams of sodium sulfate and get a 65.0% yield, how many grams of. Nh 4 no 3 + na 3 po 4 (nh 4 3 po 4 + nano 3 which. Web determine the average percent yield of mgo for the two trials. Actual yield = given in the problem or the experimental yield. Web actual yield is 14 grams, what is the percent yield? 8.2 grams b) i got a percent yield of 75% how many grams. % yield = actual yield x 100theoretical yield 1) balance the equation for. Web chemistry 1 volume 2 worksheet 12 percent yield in chemical reactions calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of. Web solutions 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. % yield =. Web up to 24% cash back theoretical yeild theoretical yield = answer to your stoich problem. This worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. _____ k2ptcl4 + __ 2 ___ nh3 g. Cucl2 + nano3 cu(no3)2 + nacl a) if 15 grams of. All of the work is shown also.docx filethechemteacher.weebly.comthe chemistry teacher on. Web solutions 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. Sn3(po4)4 + 6 na2co3 →. Web up to 24% cash back theoretical yeild theoretical yield = answer to your stoich problem. Web determine the. Grams of sodium nitrate, how much sodium. Yield is 17 g nh. Web up to 24% cash back chapter 9 percent yield practice worksheet 1. % yield = actual yield x 100theoretical yield 1) balance the equation for the reaction of iron (iii) phosphate with sodium sulfate. Actual yield = given in the problem or the experimental yield. _____ k2ptcl4 + __ 2 ___ nh3 g. 3 + 3 mg(oh) 2. List the known quantities and plan the problem. Web up to 24% cash back chapter 9 percent yield practice worksheet 1. % yield = actual yield x 100theoretical yield 1) balance the equation for the reaction of iron (iii) phosphate with sodium sulfate. Balance the equation for the reaction given below: Web chemistry 1 volume 2 worksheet 12 percent yield in chemical reactions calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of. Web up to 24% cash back theoretical yeild theoretical yield = answer to your stoich problem. 3 + 3 mg(oh). This worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. Web up to 24% cash back chapter 9 percent yield practice worksheet 1. N 2 (g) + 3 h 2 (g) 2 nh 3 (g) • 16.0 g is the actual yield (given) 28.3 g. Web determine the average percent yield of mgo for the two trials. (98.639 + 99.042)/2 = 98.8 %. Web up to 24% cash back theoretical yeild theoretical yield = answer to your stoich problem. Cucl2 + nano3 cu(no3)2 + nacl a) if 15 grams of copper (ii) chloride react with 20. % yield = actual yield x 100theoretical yield 1). Web what is the percent yield for the reaction? (98.639 + 99.042)/2 = 98.8 %. Web up to 24% cash back 1) consider the following reaction: Any yield under 100% is reasonable under the law of conservation of mass. Web chemistry 1 volume 2 worksheet 12 percent yield in chemical reactions calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of. Web up to 24% cash back chapter 9 percent yield practice worksheet 1. _____ k2ptcl4 + __ 2 ___ nh3 g. This worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. Web determine the average percent yield of mgo for the two trials. Web the percent yield of the reaction is the ratio of the actual over the theoretical yield, so rearranging this equation we can calculate the actual yield: N 2 (g) + 3 h 2 (g) 2 nh 3 (g) • 16.0 g is the actual yield (given) 28.3 g is the theoretical yield (calculated) • now that you. % yield = actual yield x 100theoretical yield 1) balance the equation for the reaction of iron (iii) phosphate with sodium sulfate. Web solutions 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and sodium phosphate. Sn3(po4)4 + 6 na2co3 →. Actual yield = given in the problem or the experimental yield. Nh 4 no 3 + na 3 po 4 (nh 4 3 po 4 + nano 3 which reactant is limiting, assuming we started with 30.0 grams of ammonium. 3 + 3 mg(oh) 2. For the balanced equation shown below, if the reaction of 20.7g caco 3 produces 6.81g cao, what is the. Cucl2 + nano3 cu(no3)2 + nacl a) if 15 grams of copper (ii) chloride react with 20. 1) balance the equation for the. % yield = actual yield x 100theoretical yield 1) balance the equation for the reaction of iron (iii) phosphate with sodium sulfate. This worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. Balance the equation for the reaction given below: Yield is 17 g nh. Web determine the average percent yield of mgo for the two trials. (98.639 + 99.042)/2 = 98.8 %. Web what is my percent yield? Web chemistry 1 volume 2 worksheet 12 percent yield in chemical reactions calculate the percent yield of a reaction that had a theoretical yield of 3.76 g and an actual yield of. For the balanced equation shown below, if the reaction of 20.7g caco 3 produces 6.81g cao, what is the. 3 + 3 mg(oh) 2. Grams of sodium nitrate, how much sodium. 3 + 3 fe 2 sb + 3. Actual yield = given in the problem or the experimental yield. Web up to 24% cash back 1) consider the following reaction: From the solved percent yield of mgo of. Web up to 24% cash back theoretical yeild theoretical yield = answer to your stoich problem.Theoretical and Percent Yield Worksheet Answers
Limiting Reactant And Percent Yield Worksheet Answers Ecopher
stoichiometry percent yield worksheet answers
Stoichiometry Percent Yield Worksheet With Answers worksheet
Percent Yield Worksheet
stoichiometry worksheet 1 answers
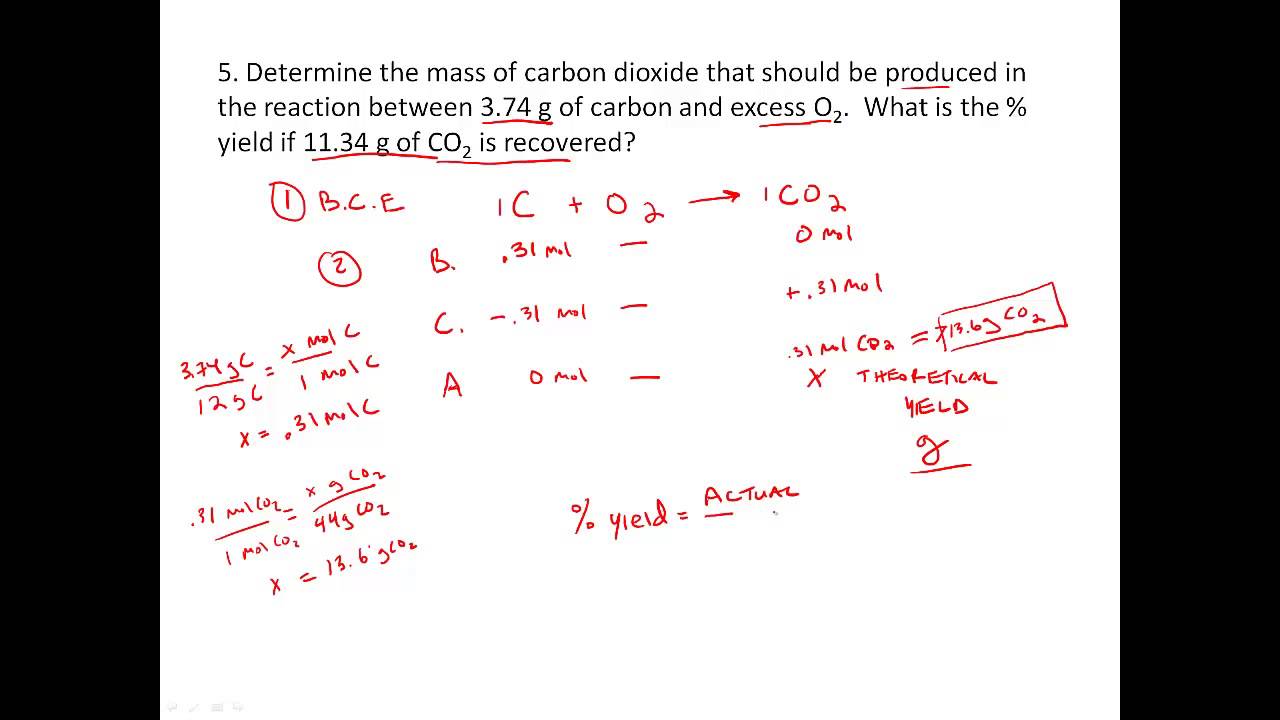
Stoichiometry practice Percent Yield problems YouTube
Theoretical and Percent Yield Worksheet Answers
14 Stoichiometry Worksheet 2 Answer Key /
Limiting Reactant And Percent Yield Practice Worksheet Answer Key
_____ K2Ptcl4 + __ 2 ___ Nh3 G.
N 2 (G) + 3 H 2 (G) 2 Nh 3 (G) • 16.0 G Is The Actual Yield (Given) 28.3 G Is The Theoretical Yield (Calculated) • Now That You.
Web What Is The Percent Yield For The Reaction?
Web Actual Yield Is 14 Grams, What Is The Percent Yield?
Related Post: